Resources
Get the latest information on
developments in ATTR-CM care
Actor portrayals.
How Attruby Works
 Filter
Filter
 Treatment
Treatment
Learn about the mechanism of action of Attruby as an FDA-approved TTR stabilizer.
ATTR-CM=transthyretin amyloid cardiomyopathy.
Long-Term Efficacy and Safety of Acoramidis in ATTR-CM: Initial Report From the Open-Label Extension of the ATTRibute-CM Trial

Judge DP, Gillmore JD, Alexander KM, et al. Circulation. 2025.
Results from an ongoing open-label extension study that was conducted to evaluate the long-term efficacy and safety of Attruby after 42 months of treatment.
Learn MoreEfficacy of Acoramidis on All-Cause Mortality and Cardiovascular Hospitalization in Transthyretin Amyloid Cardiomyopathy

Judge DP, Alexander KM, Cappelli F, et al. J Am Coll Cardiol. 2025.
This publication reports prespecified secondary and post hoc analyses from the ATTRibute-CM study to further characterize the efficacy of acoramidis on the clinical outcomes of ACM and CVH.
Learn MoreEfficacy and Safety of Acoramidis in Transthyretin Amyloid Cardiomyopathy

Gillmore JD, Judge DP, Cappelli F, et al. NEJM. 2024.
Results from ATTRibute-CM, a phase 3, double-blind, placebo-controlled study that evaluated the efficacy and safety of Attruby in patients with ATTR-CM.
Learn More
ATTR-CM: Then and Now

Learn more about recent developments in disease state awareness and diagnostic approaches in ATTR-CM.
Download PDF
Advances in ATTR-CM Imaging
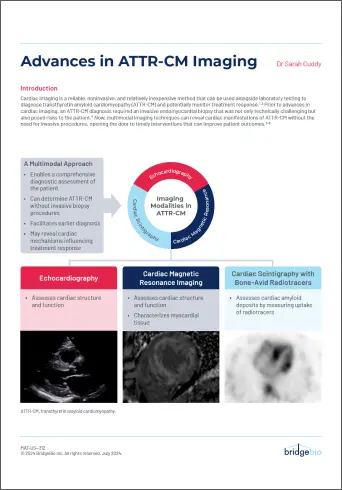
Learn more about cardiac imaging, a reliable, noninvasive, and relatively inexpensive method that can be used to help diagnose and monitor ATTR-CM.
Download PDFUnveiling ATTR-CM Among Patients with Common Cardiac Conditions
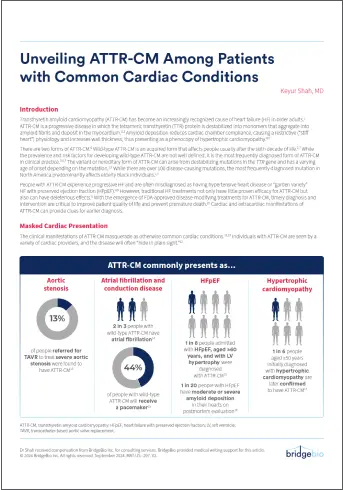
Learn more about signs and symptoms of ATTR-CM, and how to recognize these clues to make a diagnosis.
Download PDFAttruby Resources
Patient Brochure
A detailed guide with information on ATTR-CM, treatment options, and support resources to empower patients in managing their health.
Download PDF
Medisafe Flashcard
A quick reference guide offering step-by-step instructions on using Medisafe for seamless medication management.
Download PDF
Patient Resources
Visit the online hub for educational tools, support programs, and links to helpful services for patients navigating their ATTR-CM journey.
Learn MoreMOA Video
Ornare felis suspendisse ullamcorper morbi vivamus non. Enim volutpat eget mauris rutrum. Scelerisque consectetur facilisis tempus amet mattis nisi risus egestas aenean. Euismod elit faucibus pellentesque mattis adipiscing amet.
 Filter
Filter
 Treatment
Treatment
ATTR-CM=transthyretin amyloid cardiomyopathy.

Long-Term Efficacy and Safety of Acoramidis in ATTR-CM: Initial Report From the Open-Label Extension of the ATTRibute-CM Trial
Judge DP, Gillmore JD, Alexander KM, et al. Circulation. 2025.
Results from an ongoing open-label extension study that was conducted to evaluate the long-term efficacy and safety of Attruby after 42 months of treatment.
Learn More
Efficacy of Acoramidis on All-Cause Mortality and Cardiovascular Hospitalization in Transthyretin Amyloid Cardiomyopathy
Judge DP, Alexander KM, Cappelli F, et al. J Am Coll Cardiol. 2025.
This publication reports prespecified secondary and post hoc analyses from the ATTRibute-CM study to further characterize the efficacy of acoramidis on the clinical outcomes of ACM and CVH.
Learn More
Efficacy and Safety of Acoramidis in Transthyretin Amyloid Cardiomyopathy
Gillmore JD, Judge DP, Cappelli F, et al. NEJM. 2024.
Results from ATTRibute-CM, a phase 3, double-blind, placebo-controlled study that evaluated the efficacy and safety of Attruby in patients with ATTR-CM.
Learn More
ATTR-CM: Then and Now
Learn more about recent developments in disease state awareness and diagnostic approaches in ATTR-CM.
Download PDF
Advances in ATTR-CM Imaging
Learn more about cardiac imaging, a reliable, noninvasive, and relatively inexpensive method that can be used to help diagnose and monitor ATTR-CM.
Download PDF
Unveiling ATTR-CM Among Patients
with Common Cardiac Conditions
Learn more about signs and symptoms of ATTR-CM, and how to recognize these clues to make a diagnosis.
Download PDFAttruby Resources

Patient Brochure
A detailed guide with information on ATTR-CM, treatment options, and support resources to empower patients in managing their health.
Download PDF
Medisafe Flashcard
A quick reference guide offering step-by-step instructions on using Medisafe for seamless medication management.
Download PDF
Patient Resources
Visit the online hub for educational tools, support programs, and links to helpful services for patients navigating their ATTR-CM journey.
Learn MoreINDICATION
Attruby® (acoramidis) is indicated for the treatment of the cardiomyopathy of wild-type or variant transthyretin-mediated amyloidosis (ATTR-CM) in adults to reduce cardiovascular death and cardiovascular-related hospitalization.
IMPORTANT SAFETY INFORMATION
Adverse Reactions
Diarrhea (11.6% vs 7.6%) and upper abdominal pain (5.5% vs 1.4%) were
reported in patients treated with Attruby versus placebo,
respectively. The majority of these adverse reactions were mild and
resolved without drug discontinuation.
Discontinuation rates due to adverse events were similar between patients treated with Attruby versus placebo (9.3% and 8.5%, respectively).
Laboratory Tests
Mean increase in serum creatinine of 0.2 and 0.0 mg/dL and a mean
decrease in eGFR of 8.2 and 0.7 mL/min/1.73 m2 was
observed in the
adults with ATTR-CM treated with Attruby versus placebo,
respectively, at Day 28 and then stabilized. These changes were
reversible after treatment discontinuation.
Use in Specific Populations
Pregnancy & Lactation: There are no data on the use of Attruby in pregnant women. Animal data have not shown developmental risk associated with the use of Attruby in pregnancy. There are no available data on the presence of Attruby in either human or animal milk or the effects of the drug on the breastfed infant or maternal milk production.
Please see Full Prescribing Information including Patient Information.
INDICATION AND IMPORTANT
SAFETY INFORMATION
INDICATION
Attruby® (acoramidis) is indicated for the treatment of the cardiomyopathy of wild-type or variant transthyretin-mediated amyloidosis (ATTR-CM) in adults to reduce cardiovascular death and cardiovascular-related hospitalization.
IMPORTANT SAFETY INFORMATION
Adverse Reactions
Diarrhea (11.6% vs 7.6%) and upper abdominal pain (5.5% vs 1.4%) were
reported in patients treated with Attruby versus placebo,
respectively. The majority of these adverse reactions were mild and
resolved without drug discontinuation.
Discontinuation rates due to adverse events were similar between patients treated with Attruby versus placebo (9.3% and 8.5%, respectively).
Laboratory Tests
Mean increase in serum creatinine of 0.2 and 0.0 mg/dL and a mean
decrease in eGFR of 8.2 and 0.7 mL/min/1.73 m2 was
observed in the
adults with ATTR-CM treated with Attruby versus placebo,
respectively, at Day 28 and then stabilized. These changes were
reversible after treatment discontinuation.
Use in Specific Populations
Pregnancy & Lactation: There are no data on the use of Attruby in pregnant women. Animal data have not shown developmental risk associated with the use of Attruby in pregnancy. There are no available data on the presence of Attruby in either human or animal milk or the effects of the drug on the breastfed infant or maternal milk production.
Please see Full Prescribing Information including Patient Information.

