Attruby is specifically designed to deliver
near-complete
TTR stabilization1-3
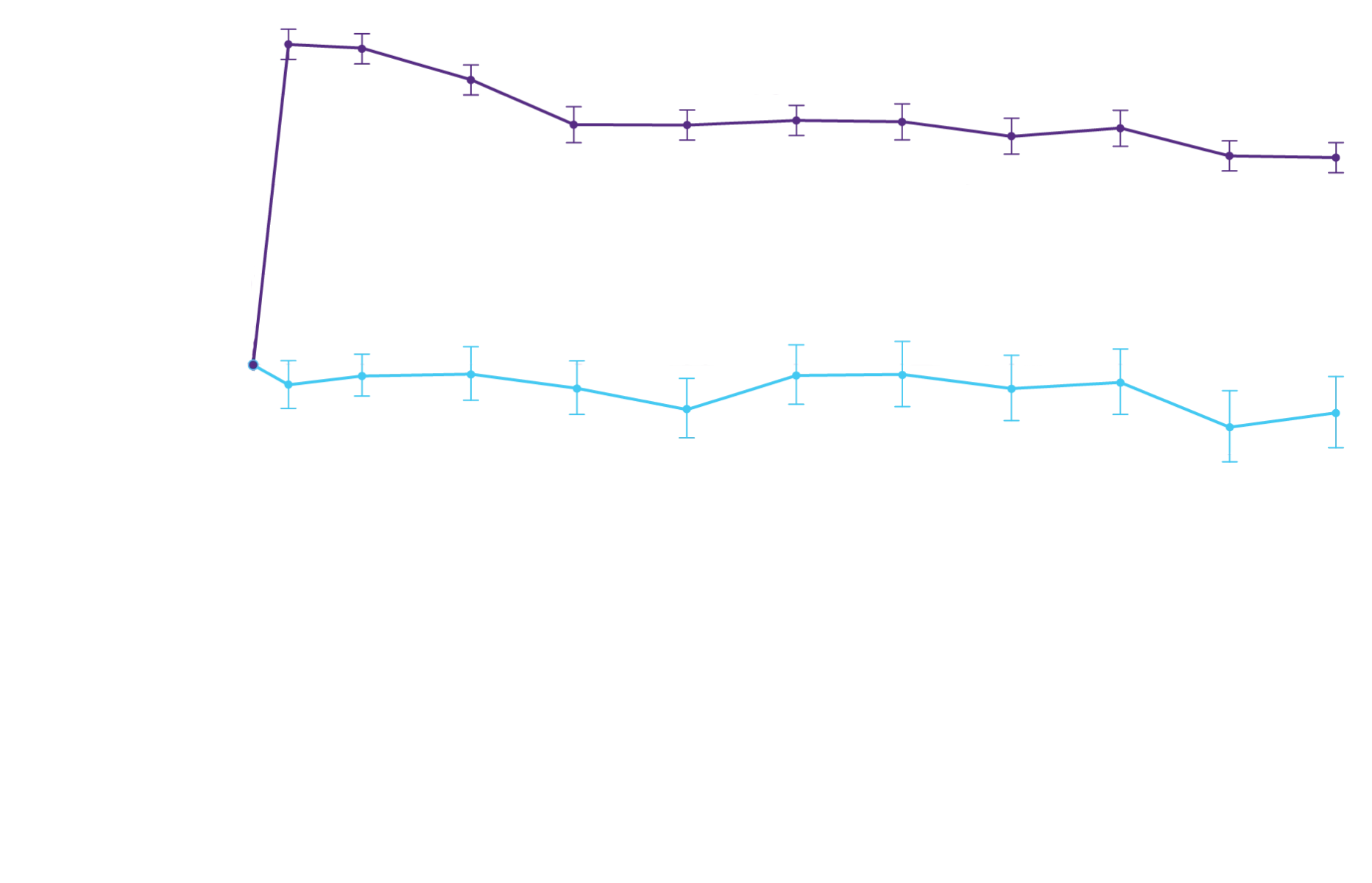
Watch >90%
Stabilization in
Action

Low rates of discontinuations due to adverse drug reactions and convenient dosing with Attruby1
See Dosing + Safety
AE=adverse event; ATTR-CM=transthyretin amyloid cardiomyopathy; GI=gastrointestinal; TTR=transthyretin.
References: 1. Attruby. Prescribing information. BridgeBio, Inc.; 2024.
2. Gillmore JD, Judge DP, Cappelli F, et al. Efficacy and safety of acoramidis in transthyretin amyloid cardiomyopathy. N Engl J Med. 2024;390(2):132-142. doi:10.1056/NEJMoa2305434
3. Hammarström P, Jiang X, Hurshman AR, Powers ET, Kelly JW. Sequence-dependent denaturation energetics: a major determinant in amyloid disease diversity. Proc Natl Acad Sci USA. 2002;99(Suppl 4):16427-16432. doi:10.1073/pnas.202495199
4. Liz MA, Coelho T, Bellotti V, Fernandez-Arias MI, Mallaina P, Obici L. A narrative review of the role of transthyretin in health and disease. Neurol Ther. 2020;9(2):395-402. doi:10.1007/s40120-020-00217-0
5. Vieira M, Saraiva MJ. Transthyretin: a multifaceted protein. Biomol Concepts. 2014;5(1):45-54. doi:10.1515/bmc-2013-0038
6. Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(22):2872-2891. doi:10.1016/j.jacc.2019.04.003
7. Kittleson MM, Maurer MS, Ambardekar AV, et al. Cardiac amyloidosis: evolving diagnosis and management: a scientific statement from the American Heart Association. Circulation. 2020;142(1): e7-e22. doi:10.1161/CIR.0000000000000792
8. Witteles RM, Bokhari S, Damy T, et al. Screening for transthyretin amyloid cardiomyopathy in everyday practice. JACC Heart Fail. 2019;7(8):709-716. doi:10.1016/j.jchf.2019.04.010
9. Morfino P, Aimo A, Vergaro G, et al. Transthyretin stabilizers and seeding inhibitors as therapies for amyloid transthyretin cardiomyopathy. Pharmaceutics. 2023;15(4):1129. doi:10.3390/pharmaceutics15041129
10. Gertz MA, Benson MD, Dyck PJ, et al. Diagnosis, prognosis, and therapy of transthyretin amyloidosis. J Am Coll Cardiol. 2015;66(21):2451-2466. doi:10.1016/j.jacc.2015.09.075
11. Angueira A, Abramowitz SA, Levin MG. Unfolding the link between transthyretin stability and survival. JAMA Cardiol. Published online December 4, 2024. doi:10.1001/jamacardio.2024.4112
12. Christoffersen M, Greve AM, Hornstrup LS, Frikke-Schmidt R, Nordestgaard BG, Tybjaerg-Hansen A. Transthyretin tetramer destabilization and increased mortality in the general population. JAMA Cardiol. Published online December 4, 2024. doi:10.1001/jamacardio.2024.4102
13. Hanson JLS, Arvanitis M, Koch CM, et al. Use of serum transthyretin as a prognostic indicator and predictor of outcome in cardiac amyloid disease associated with wild-type transthyretin. Circ Heart Fail. 2018;11(2): e004000. doi:10.1161/CIRCHEARTFAILURE.117.004000
14. Greve AM, Christoffersen M, Frikke-Schmidt R, Nordestgaard BG, Tybjaerg-Hansen A. Association of low plasma transthyretin concentration with risk of heart failure in the general population. JAMA Cardiol. 2021;6(3):258-266. doi:10.1001/jamacardio.2020.5969
15. Data on file. BridgeBio, Inc.; 2024.
Indication and Important safety information
INDICATION
Attruby® (acoramidis) is indicated for the treatment of the cardiomyopathy of wild-type or variant transthyretin-mediated amyloidosis (ATTR-CM) in adults to reduce cardiovascular death and cardiovascular-related hospitalization.
IMPORTANT SAFETY INFORMATION
Adverse Reactions
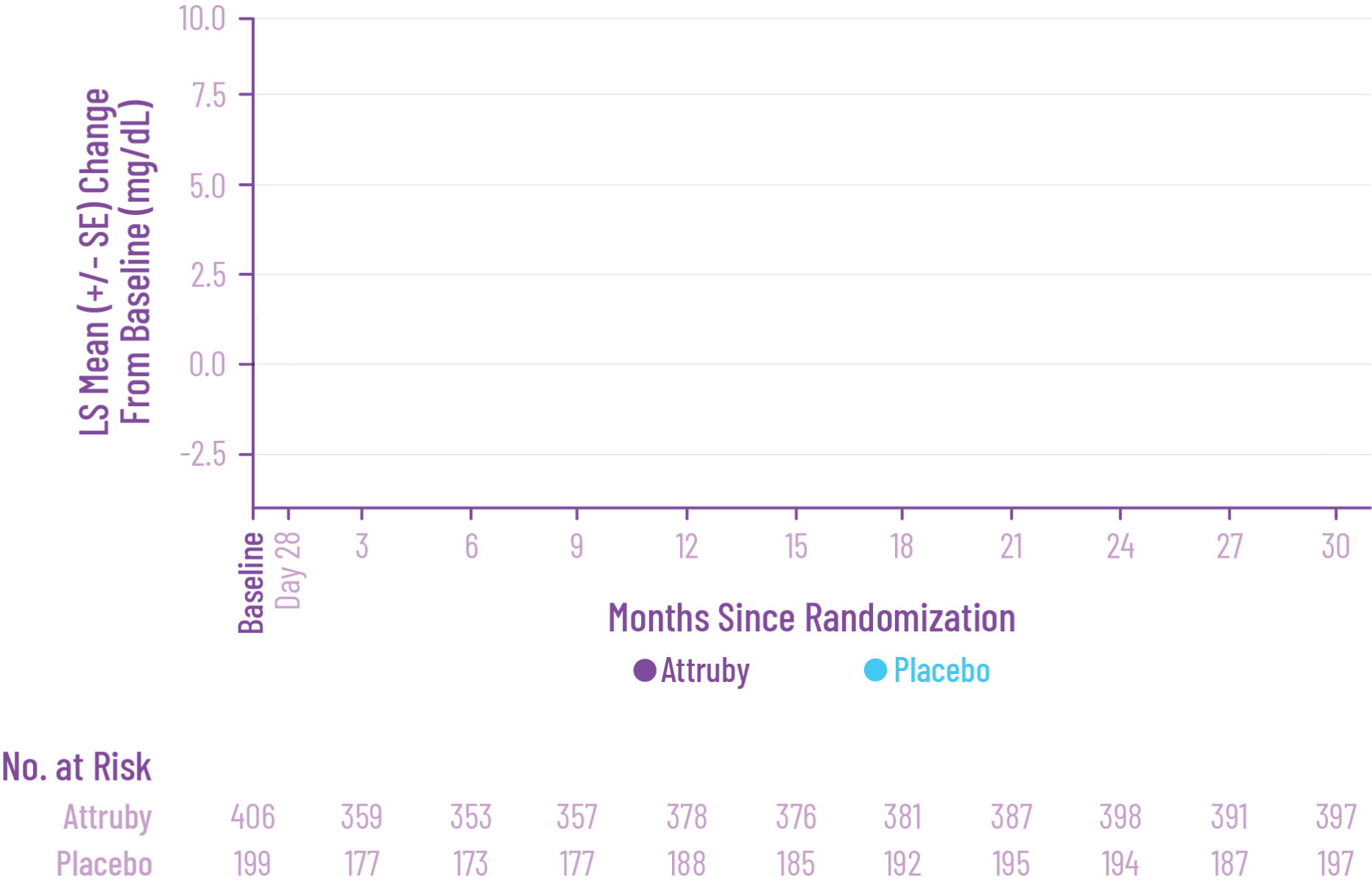
Diarrhea (11.6% vs 7.6%) and upper abdominal pain (5.5% vs 1.4%) were
reported in patients treated with Attruby versus placebo,
respectively. The majority of these adverse reactions were mild and
resolved without drug discontinuation.
Discontinuation rates due to adverse events were similar between patients treated with Attruby versus placebo (9.3% and 8.5%, respectively).
Laboratory Tests
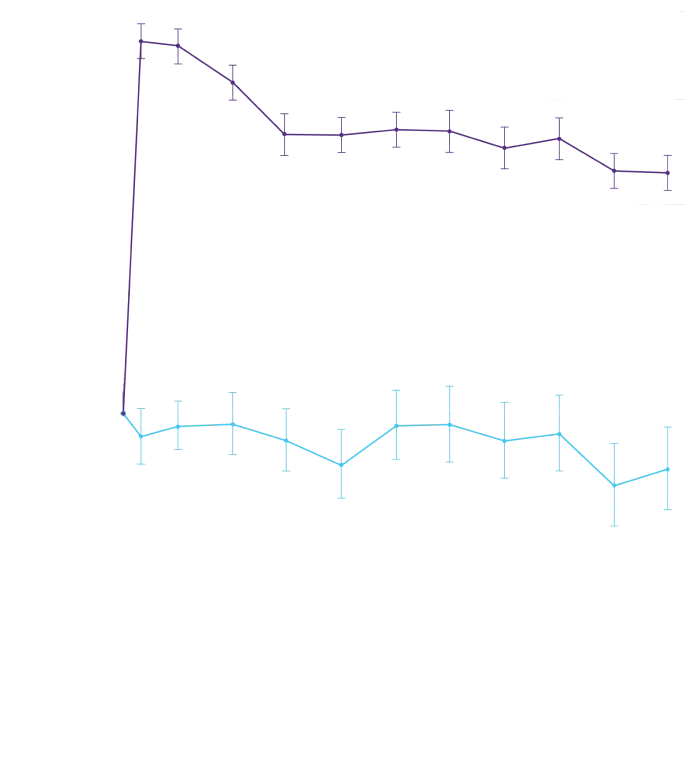
Mean increase in serum creatinine of 0.2 and 0.0 mg/dL and a mean
decrease in eGFR of 8.2 and 0.7 mL/min/1.73 m2 was
observed in the
adults with ATTR-CM treated with Attruby versus placebo,
respectively, at Day 28 and then stabilized. These changes were
reversible after treatment discontinuation.
Use in Specific Populations
Pregnancy & Lactation: There are no data on the use of Attruby in pregnant women. Animal data have not shown developmental risk associated with the use of Attruby in pregnancy. There are no available data on the presence of Attruby in either human or animal milk or the effects of the drug on the breastfed infant or maternal milk production.
Please see Full Prescribing Information including Patient Information.
INDICATION AND IMPORTANT
SAFETY INFORMATION
INDICATION
Attruby® (acoramidis) is indicated for the treatment of the cardiomyopathy of wild-type or variant transthyretin-mediated amyloidosis (ATTR-CM) in adults to reduce cardiovascular death and cardiovascular-related hospitalization.
IMPORTANT SAFETY INFORMATION
Adverse Reactions
Diarrhea (11.6% vs 7.6%) and upper abdominal pain (5.5% vs 1.4%) were
reported in patients treated with Attruby versus placebo,
respectively. The majority of these adverse reactions were mild and
resolved without drug discontinuation.
Discontinuation rates due to adverse events were similar between patients treated with Attruby versus placebo (9.3% and 8.5%, respectively).
Laboratory Tests
Mean increase in serum creatinine of 0.2 and 0.0 mg/dL and a mean
decrease in eGFR of 8.2 and 0.7 mL/min/1.73 m2 was
observed in the
adults with ATTR-CM treated with Attruby versus placebo,
respectively, at Day 28 and then stabilized. These changes were
reversible after treatment discontinuation.
Use in Specific Populations
Pregnancy & Lactation: There are no data on the use of Attruby in pregnant women. Animal data have not shown developmental risk associated with the use of Attruby in pregnancy. There are no available data on the presence of Attruby in either human or animal milk or the effects of the drug on the breastfed infant or maternal milk production.
Please see Full Prescribing Information including Patient Information.