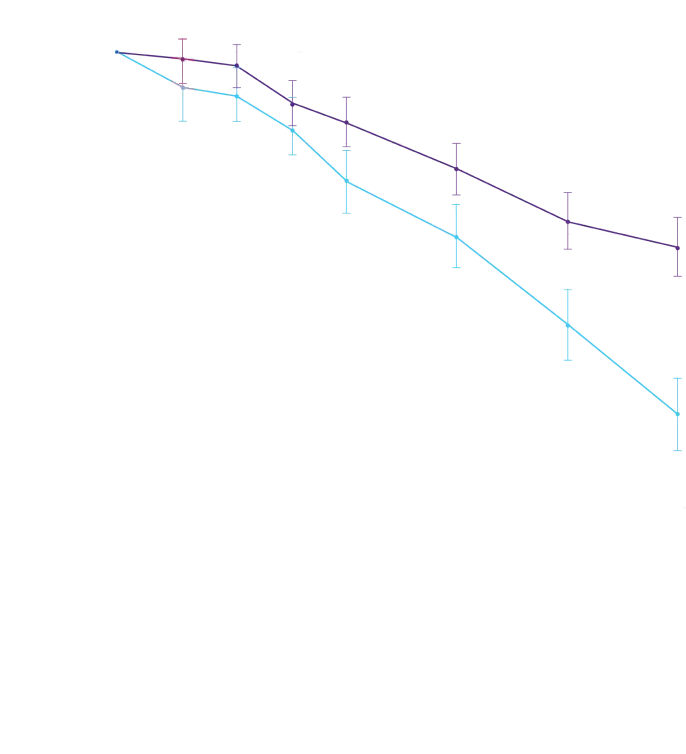
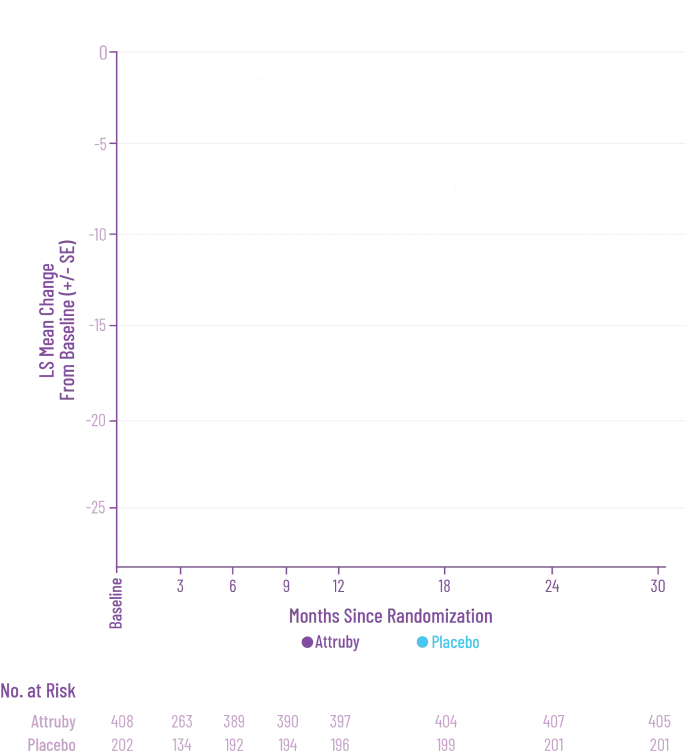
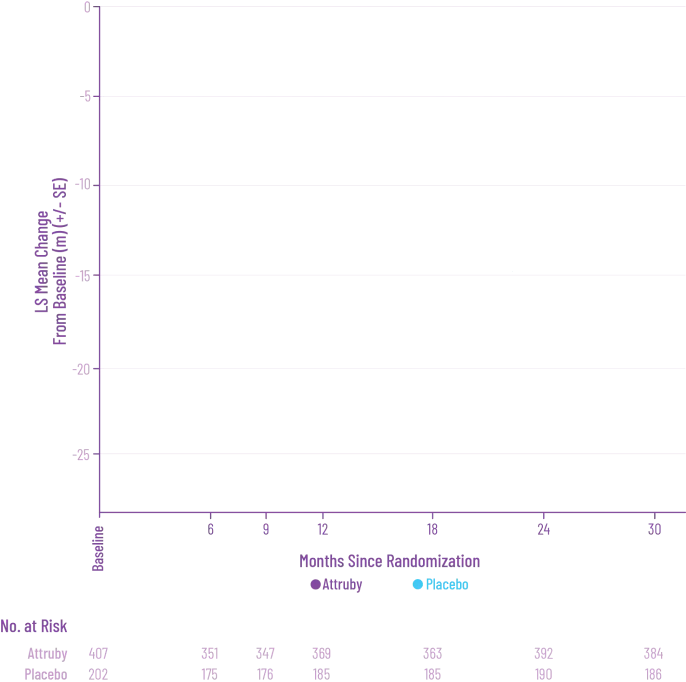
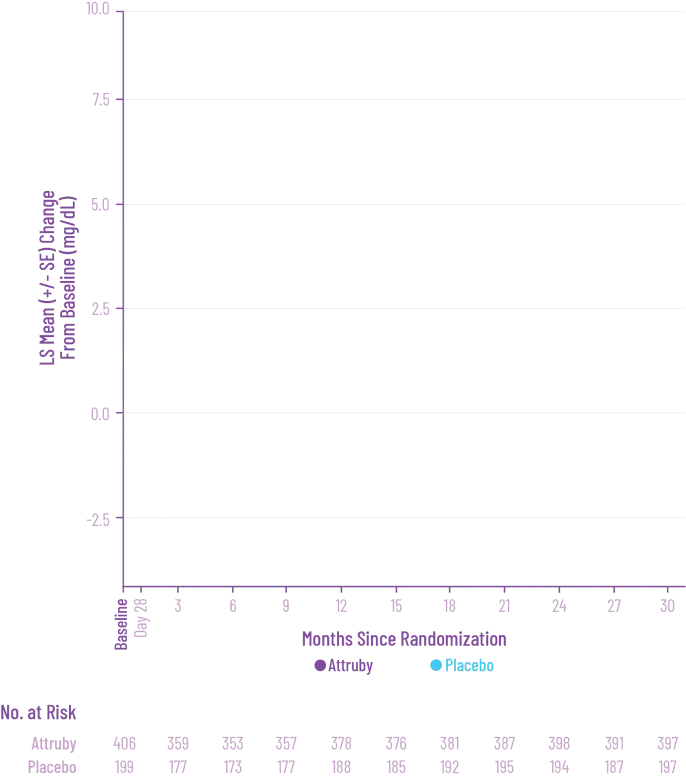
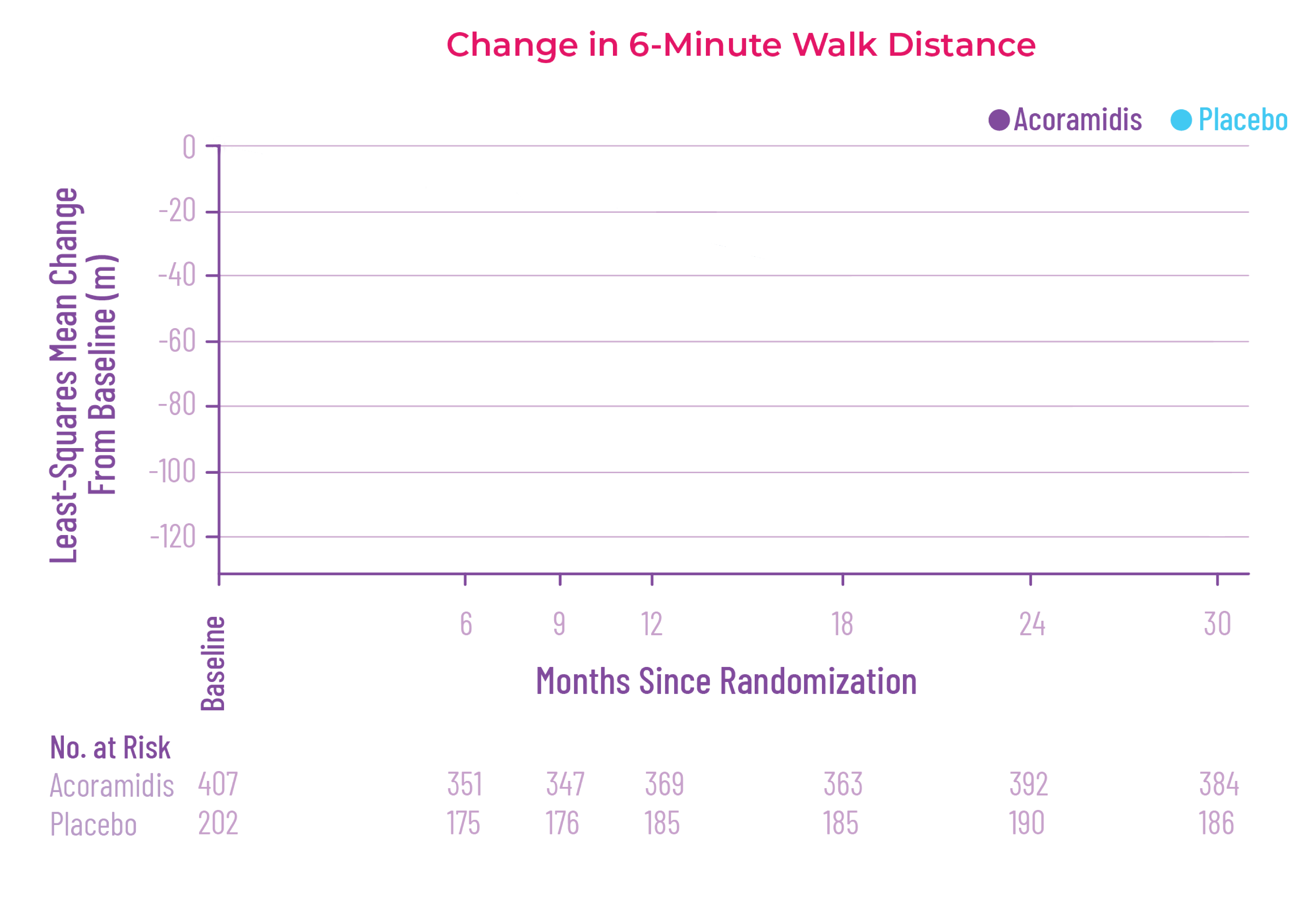
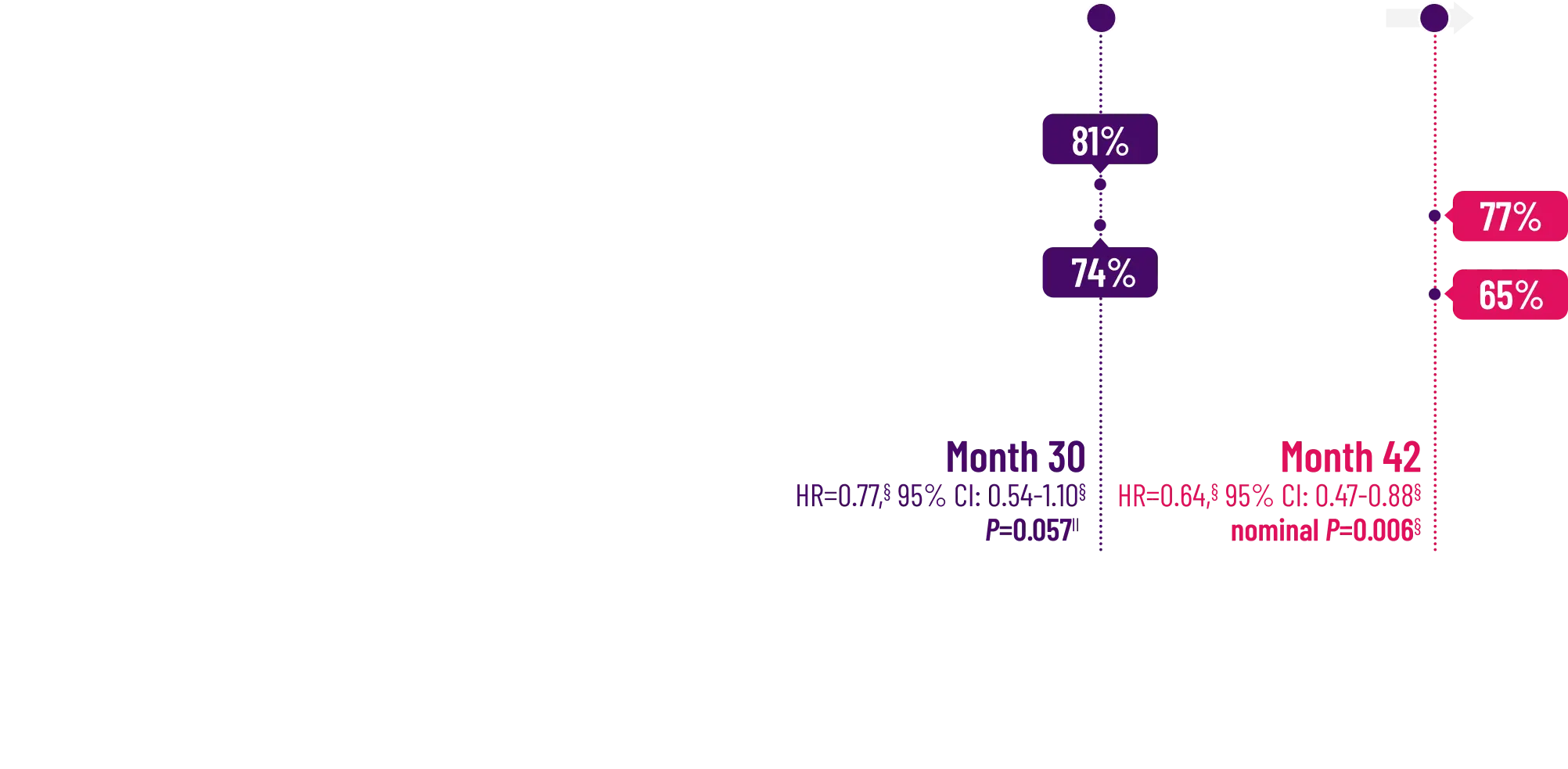
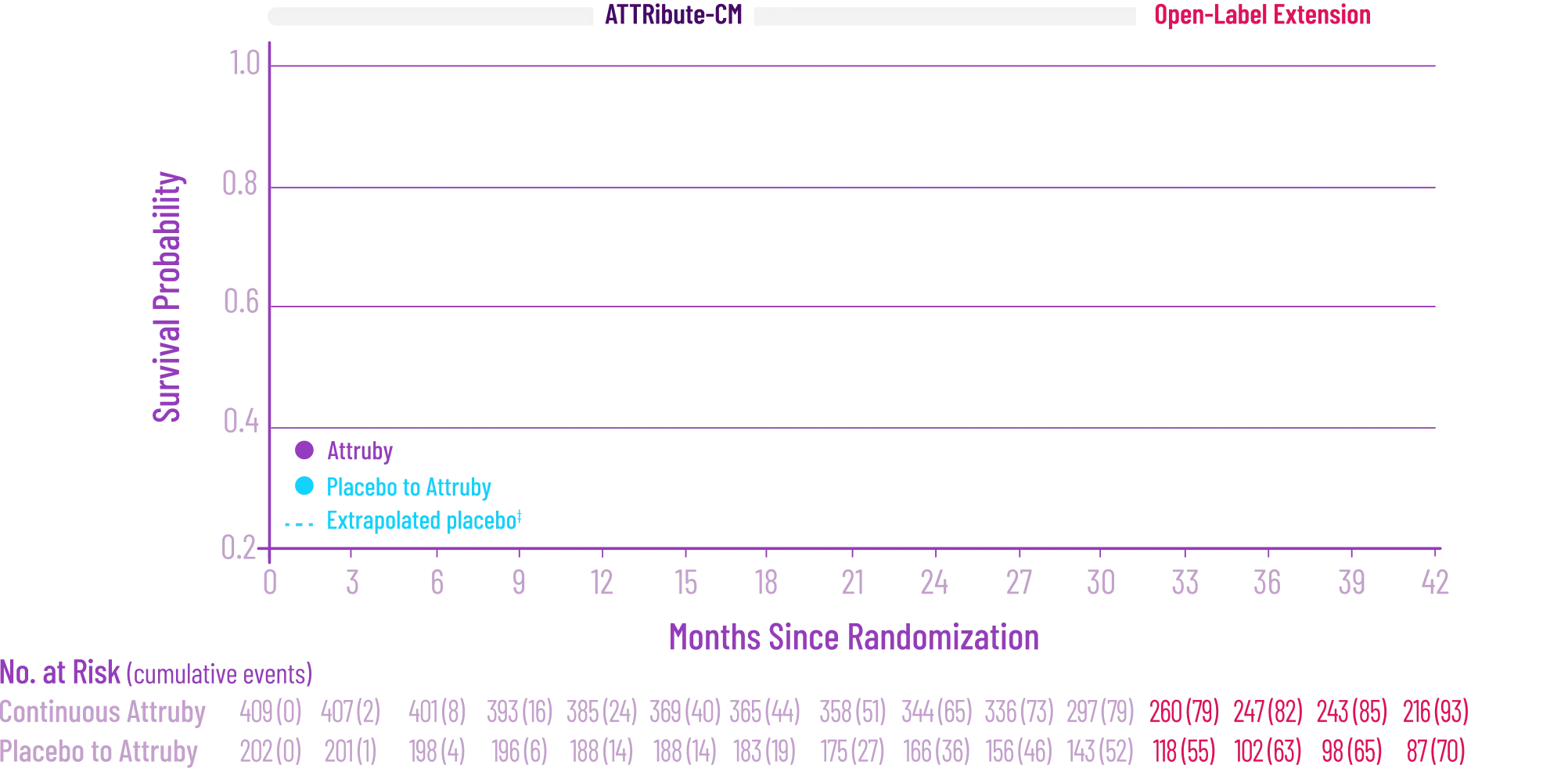
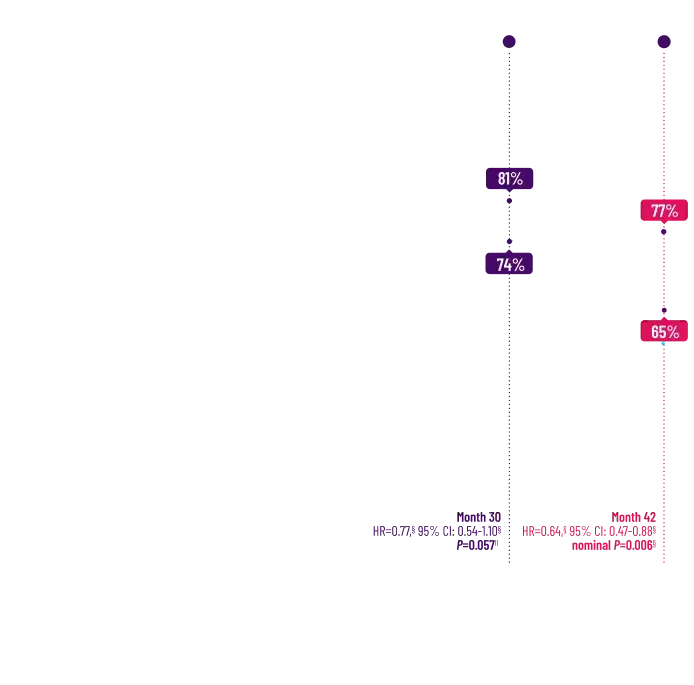
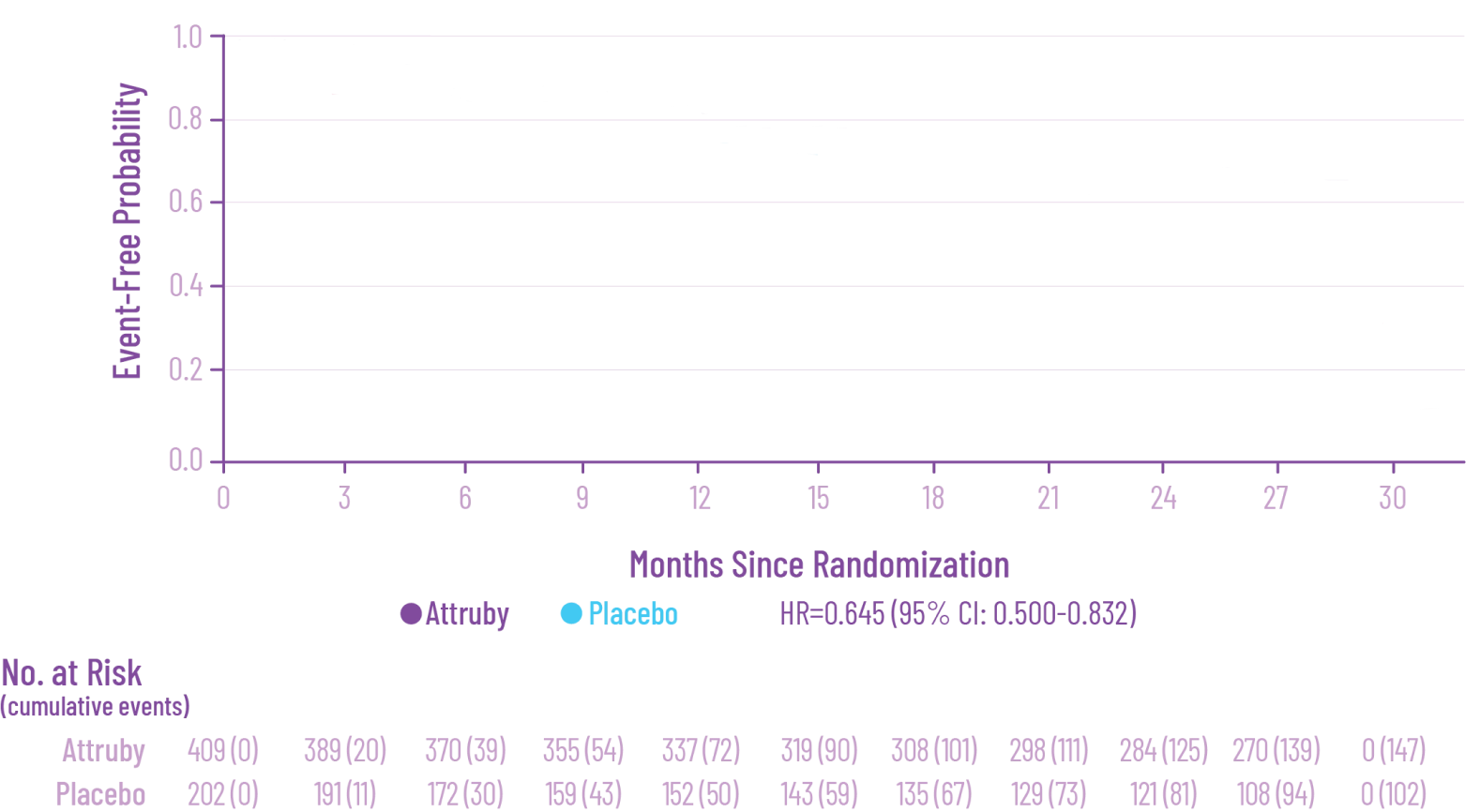
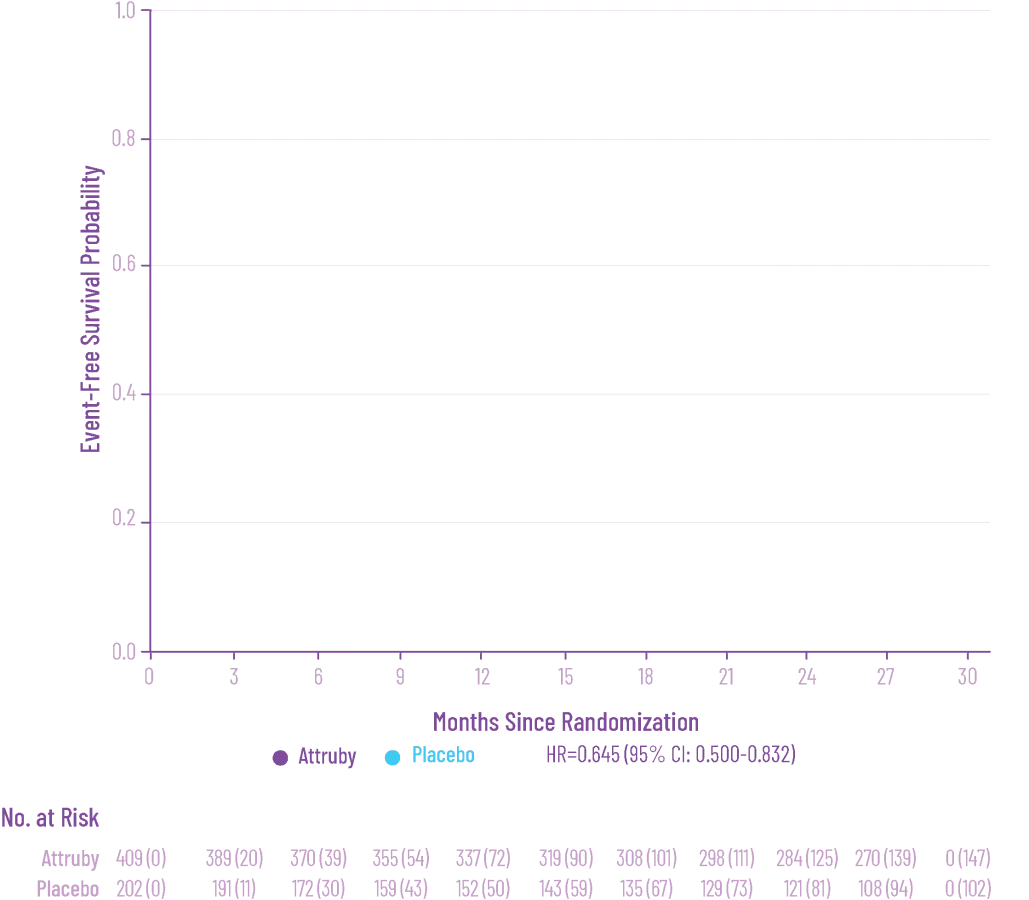
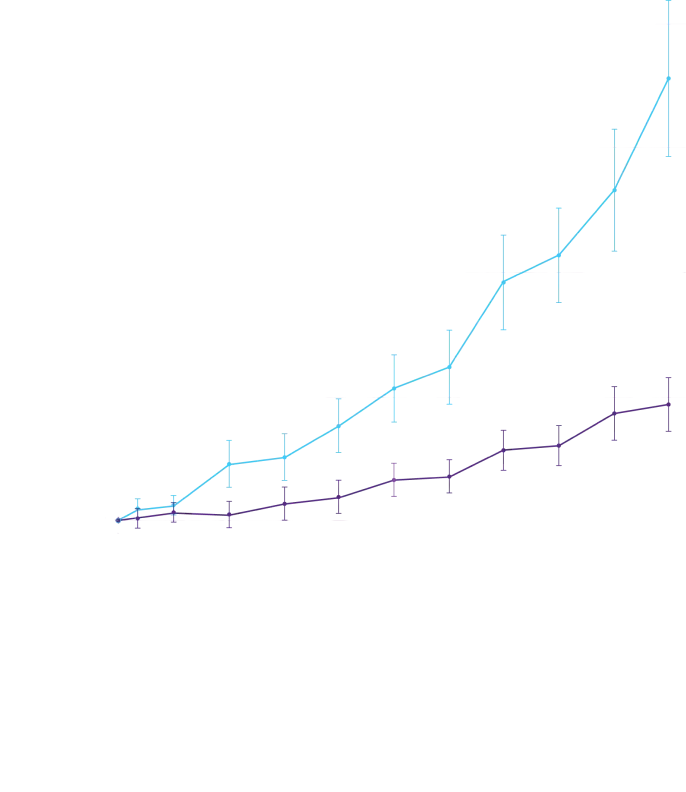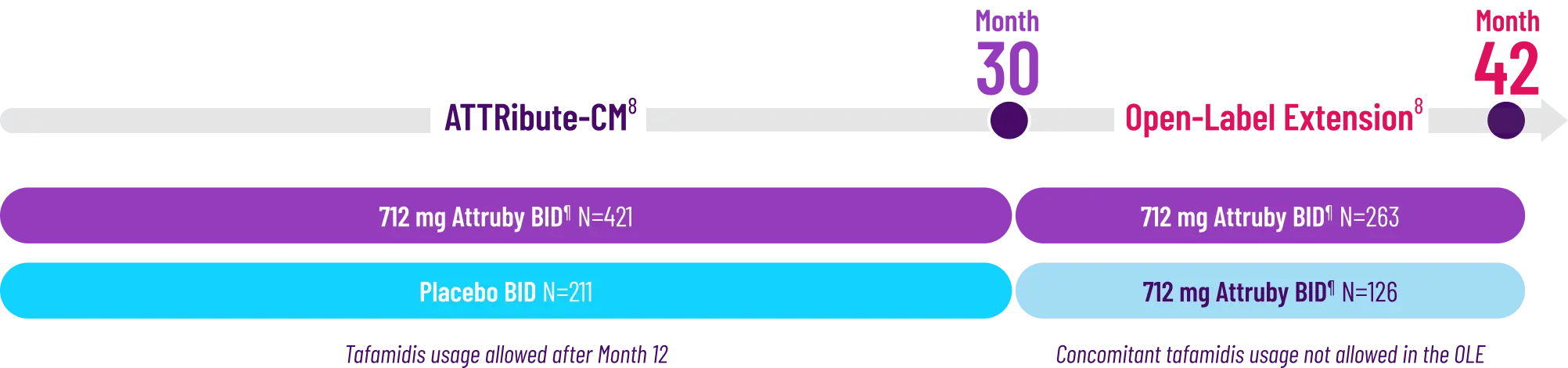Attruby was proven to significantly reduce the combination of all-cause
mortality and CV-related hospitalizations at 30 months1,2
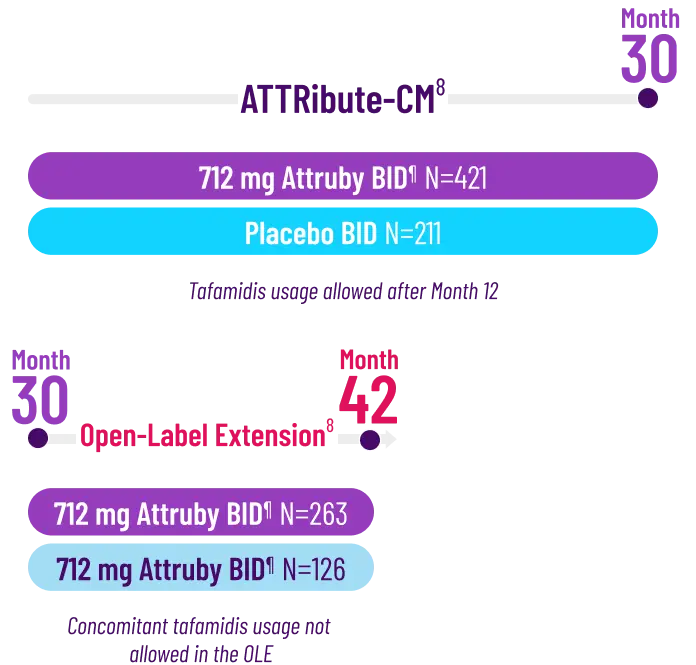
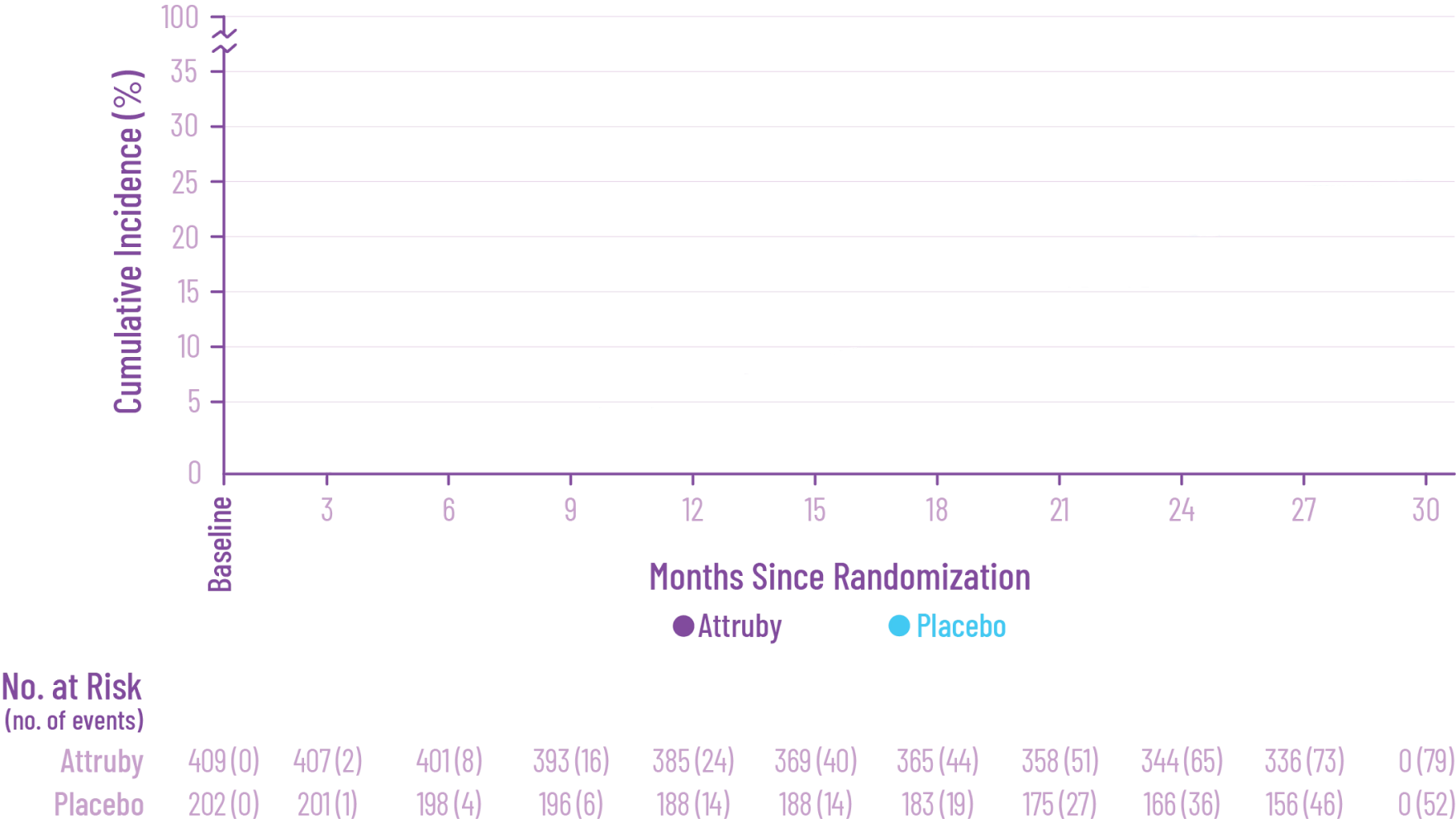
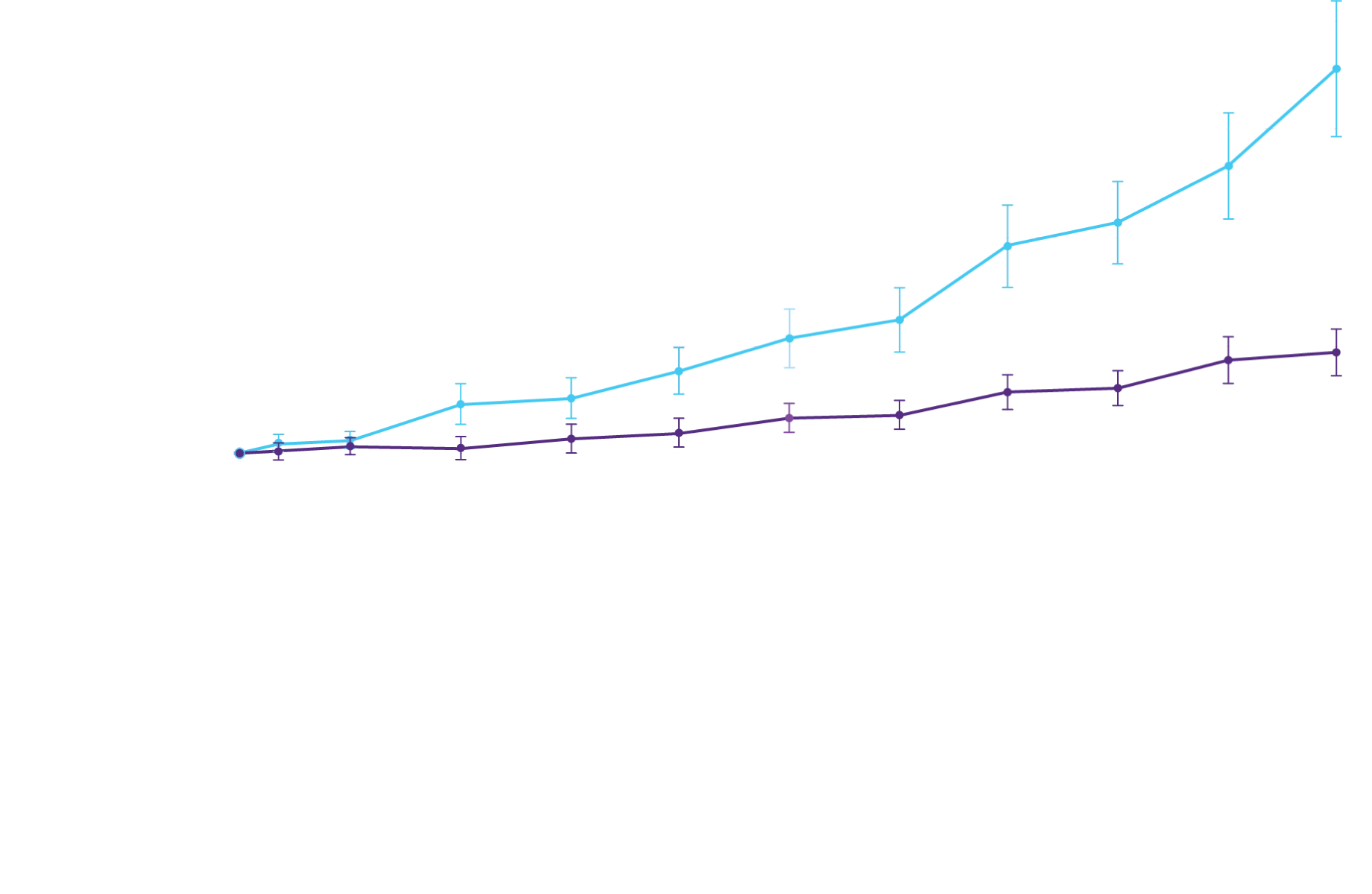
The primary composite endpoint included all-cause mortality (ACM) and cumulative
frequency of cardiovascular-related hospitalizations (CVH) over 30 months
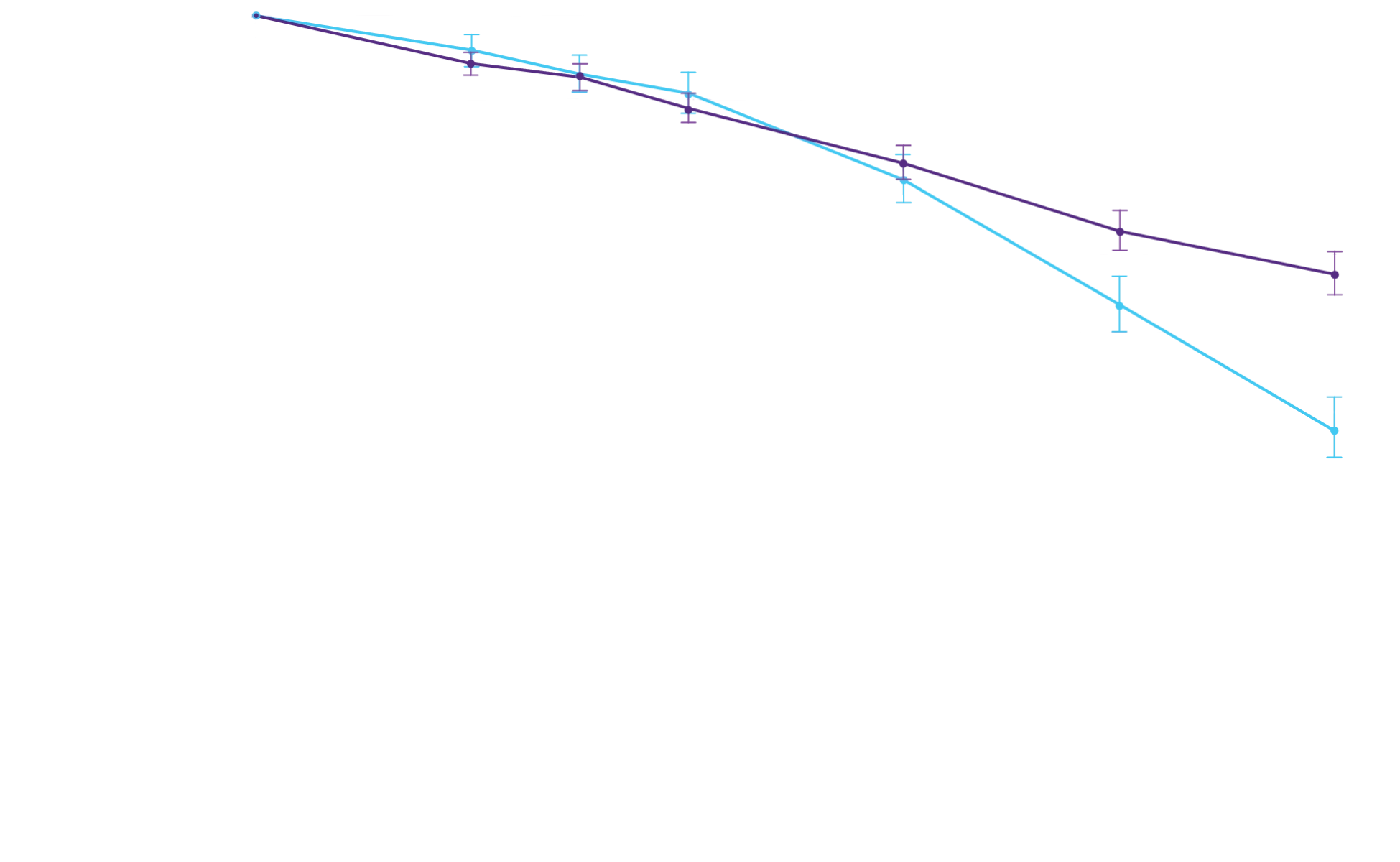
All-cause mortality
Frequency of CV-related
hospitalizations
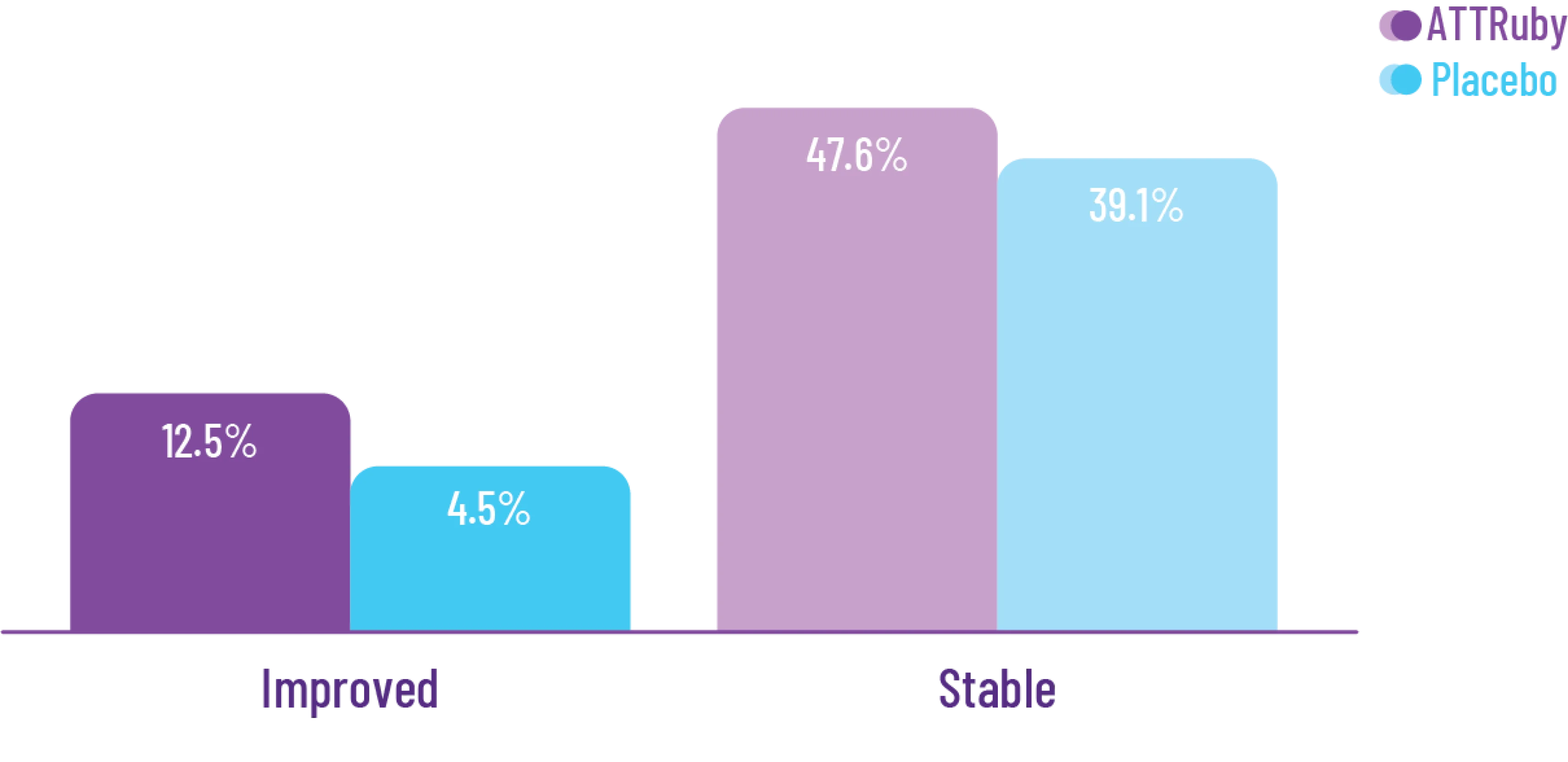
Deliver meaningful reductions in ACM and CVH for today's patient population
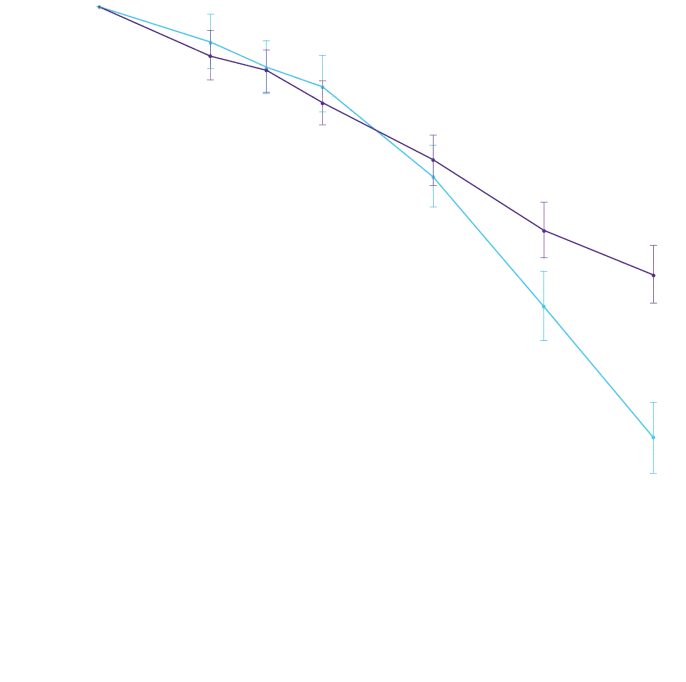
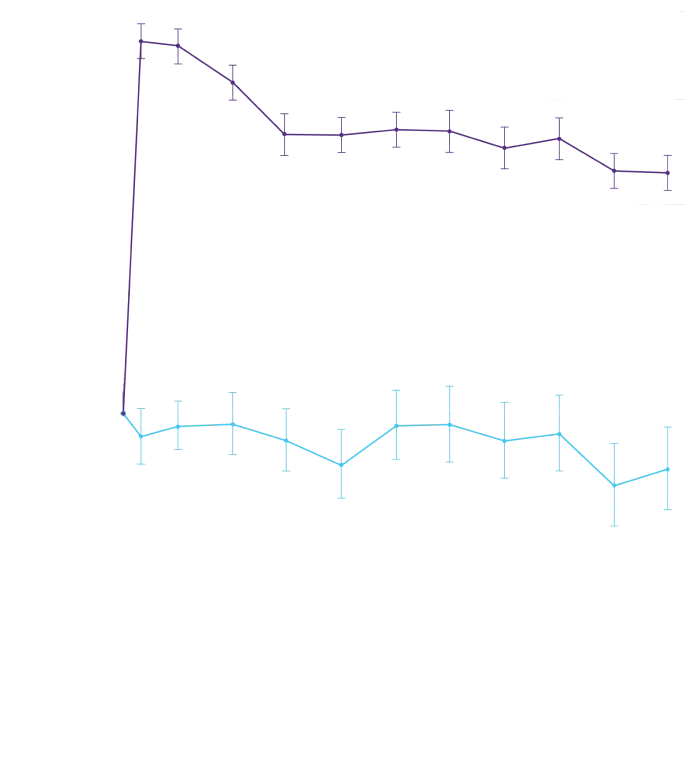
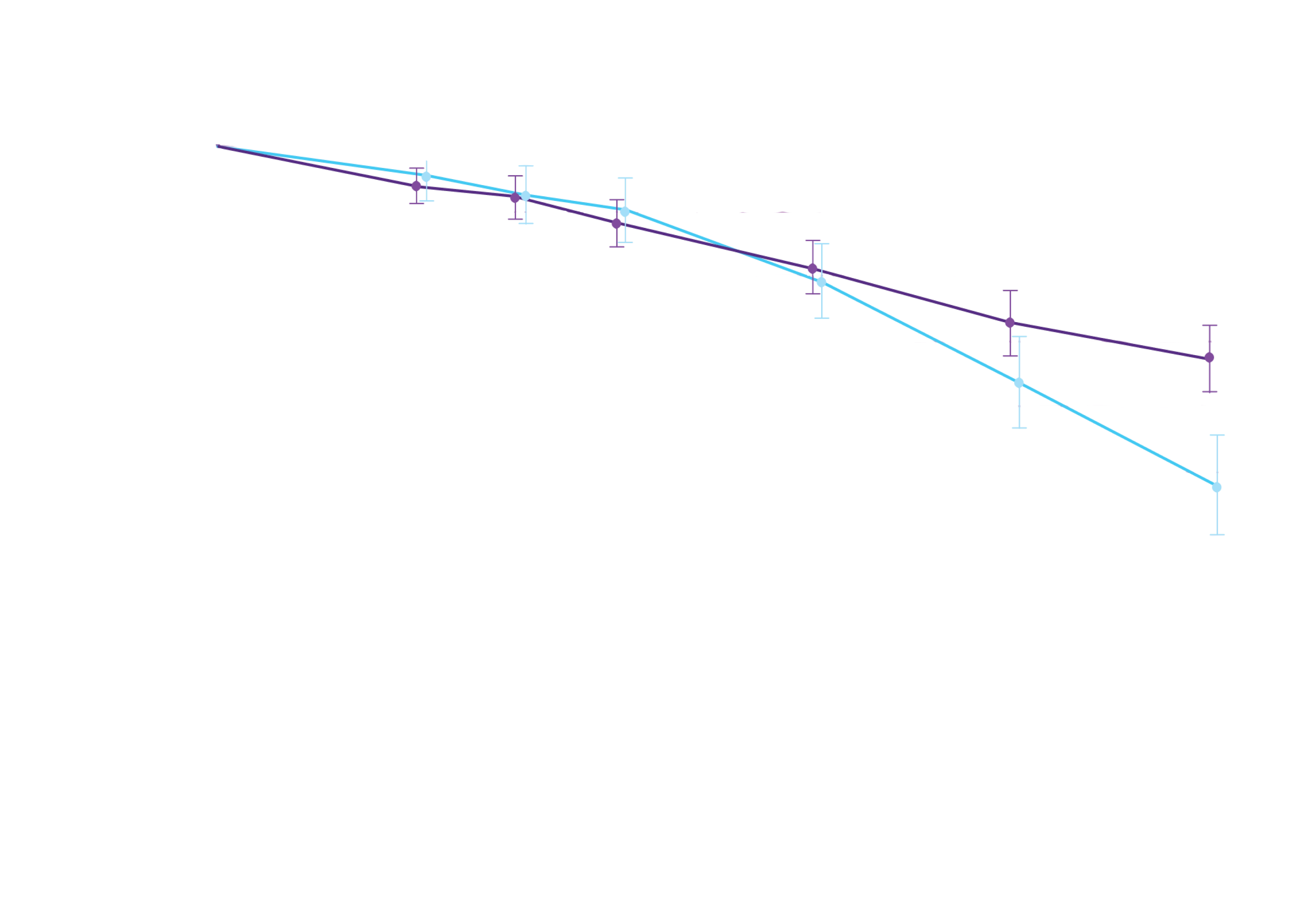
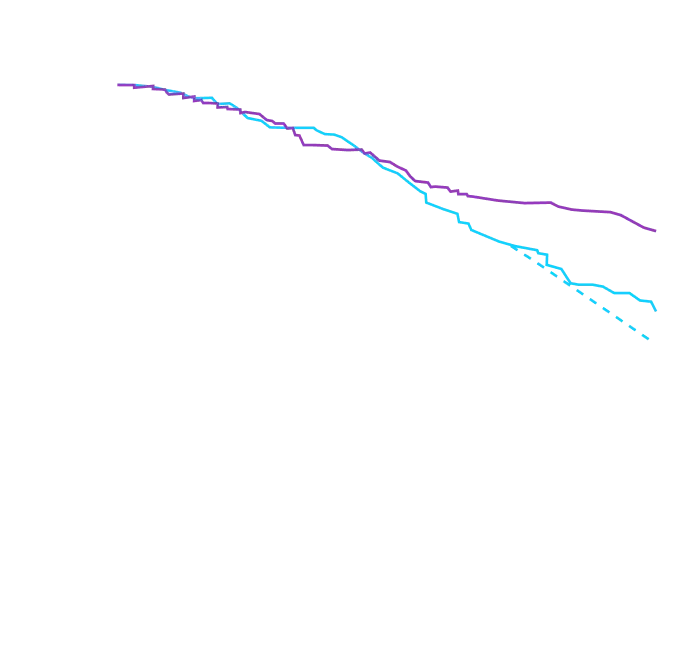
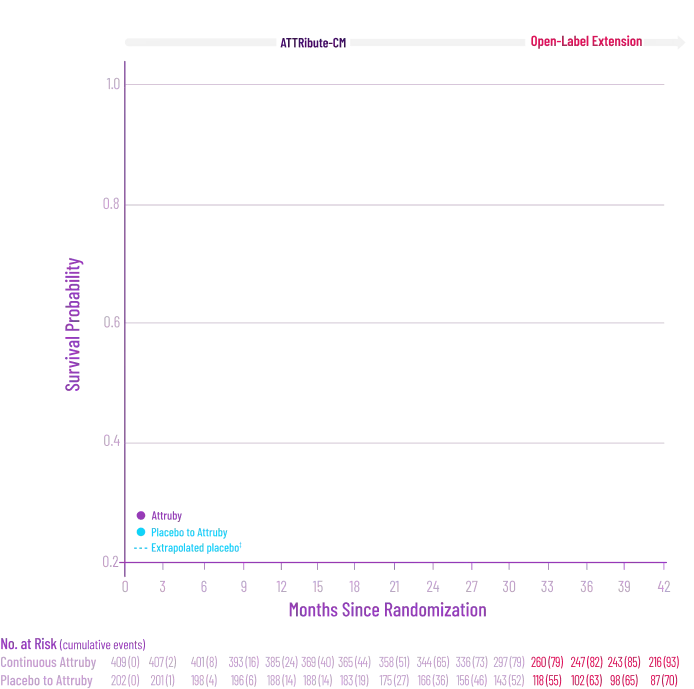
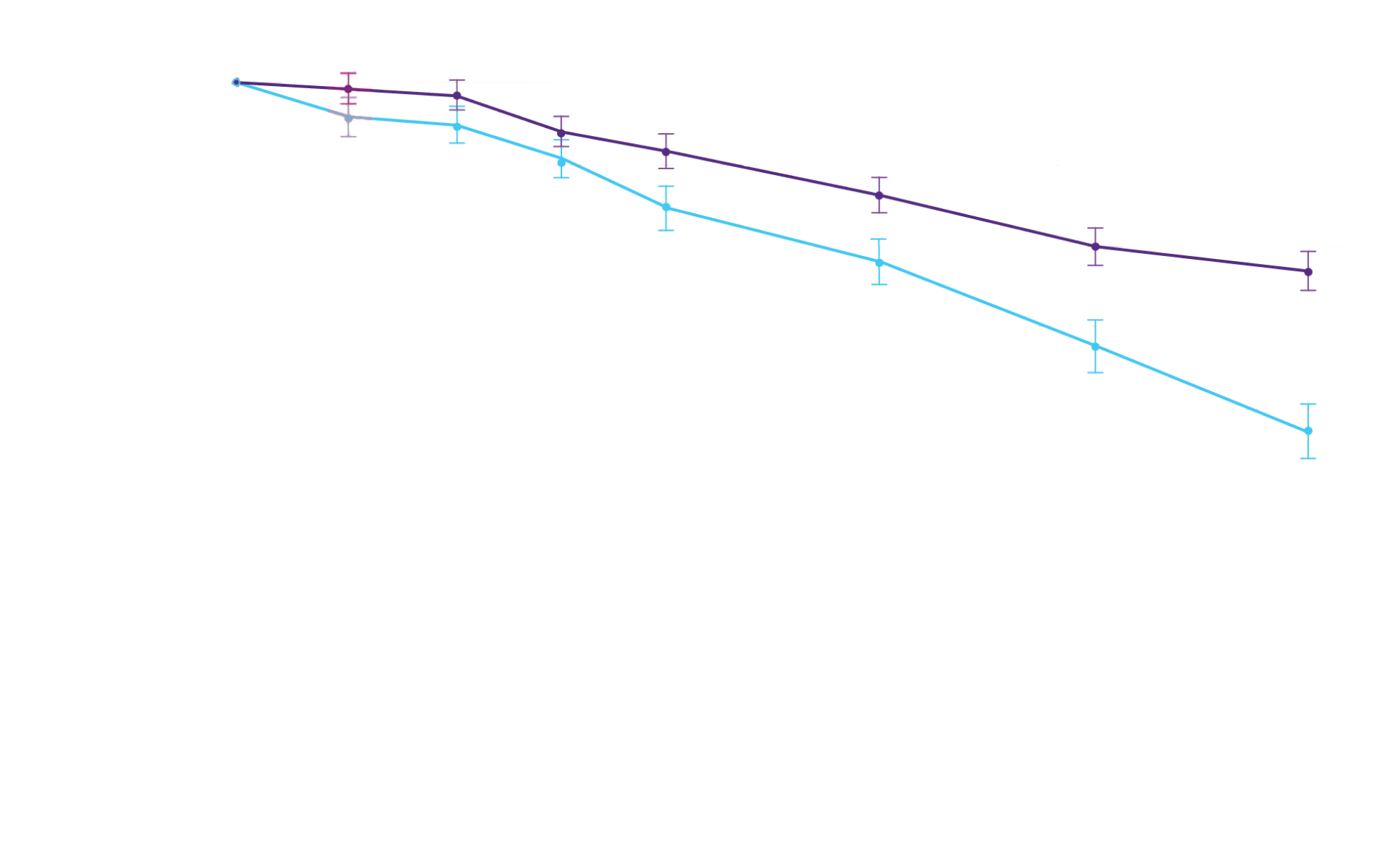
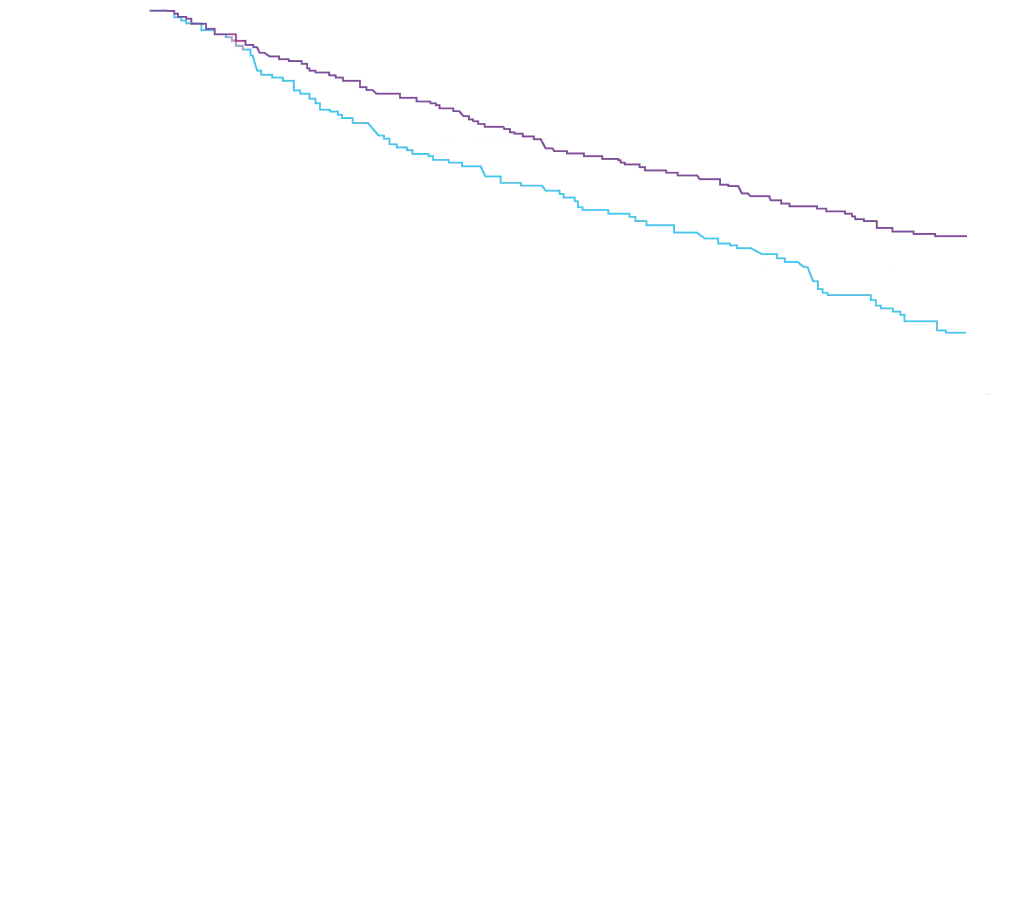
Attruby showed a rapid observable
difference that lasted over time1
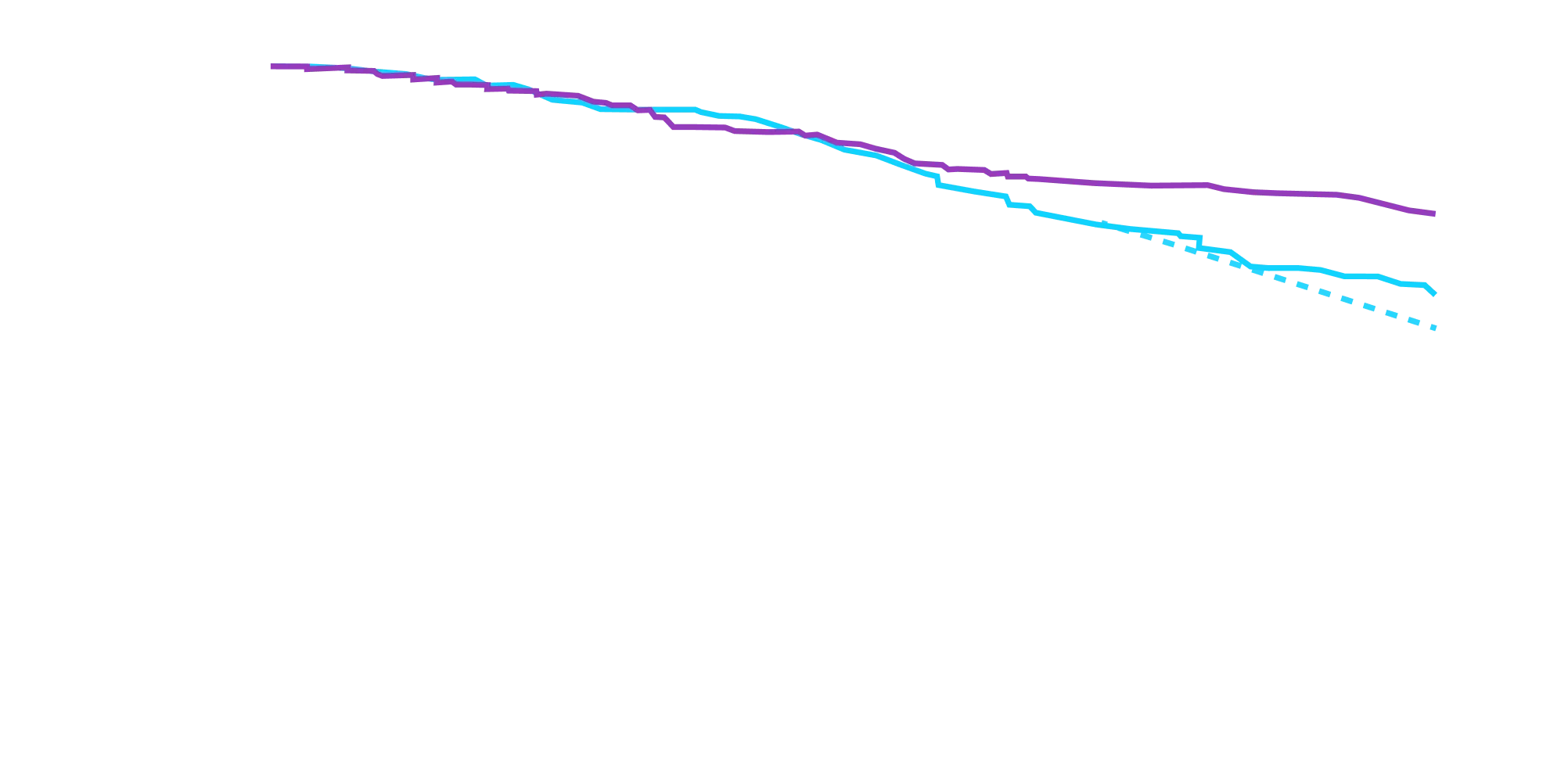
In as early as 3 months, the time to first event (ACM or CVH) curves
began separating and diverged through 30 months
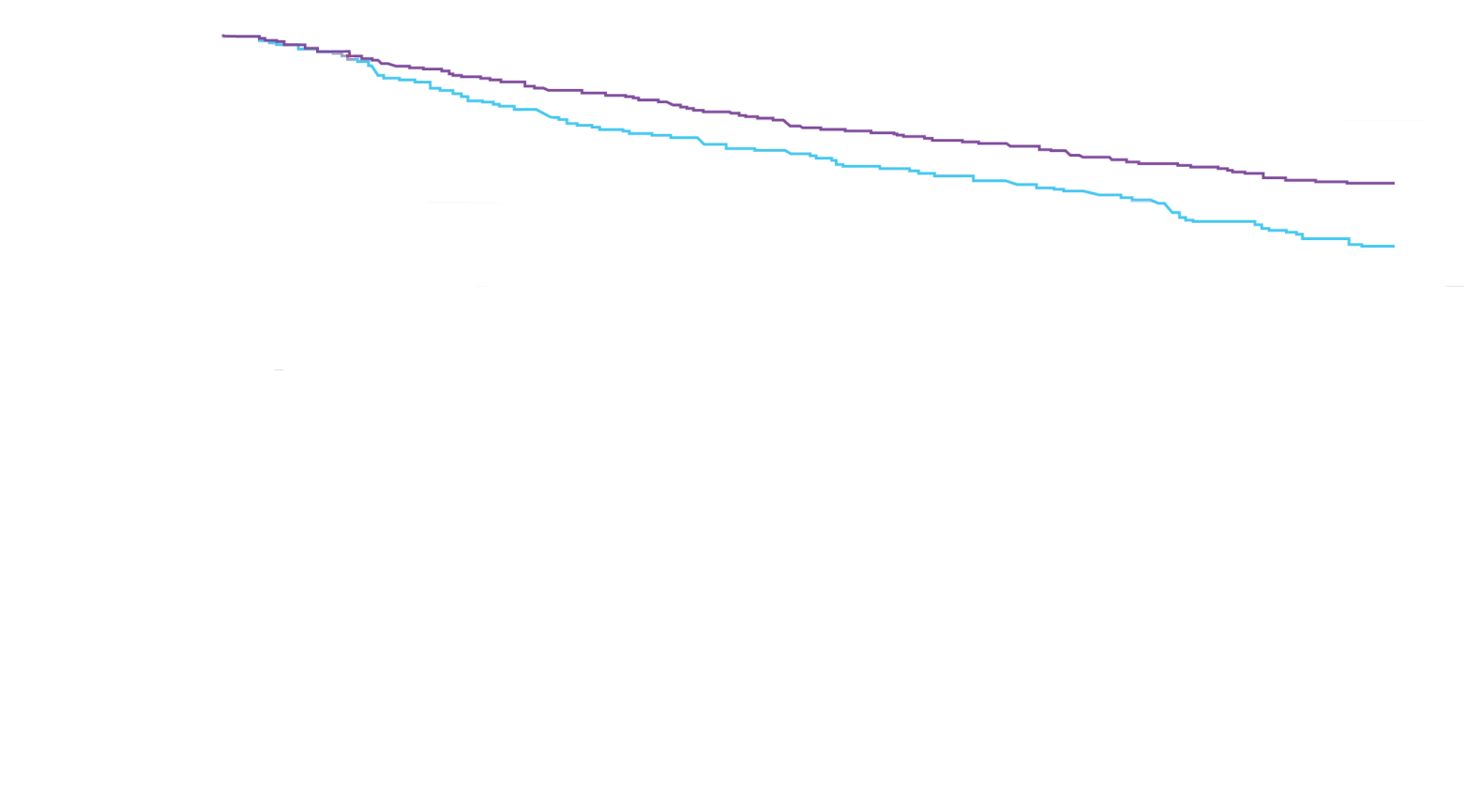
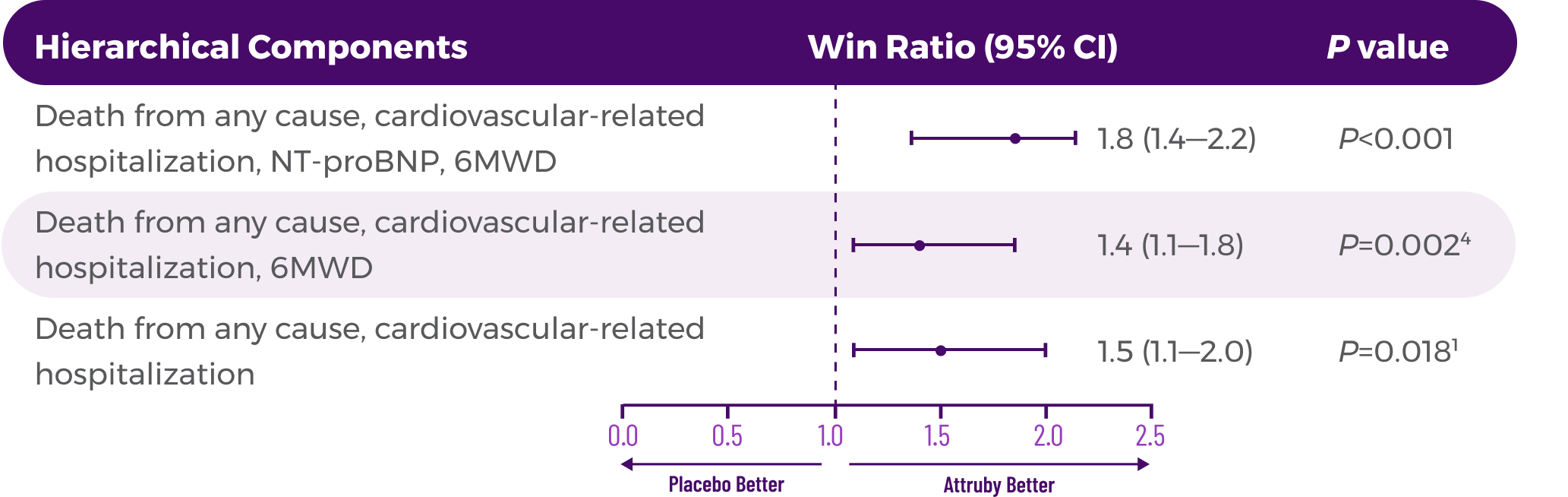
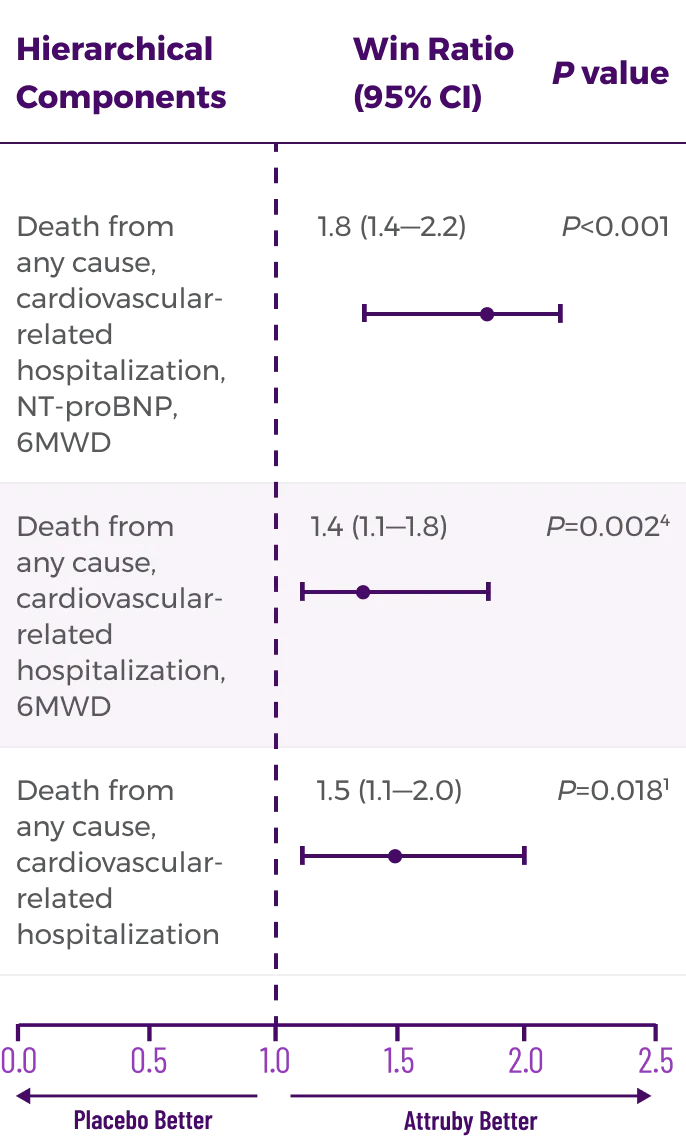
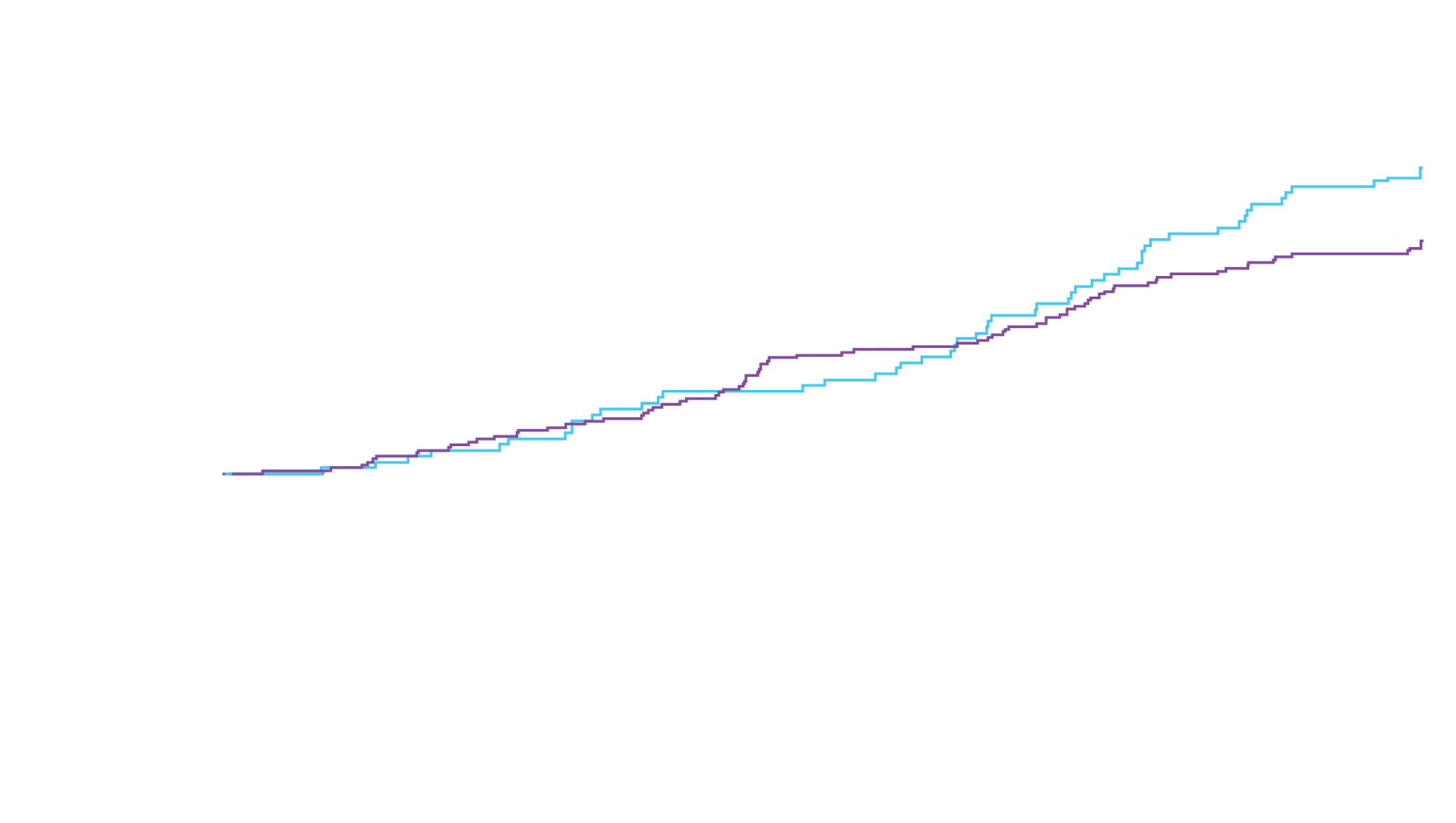
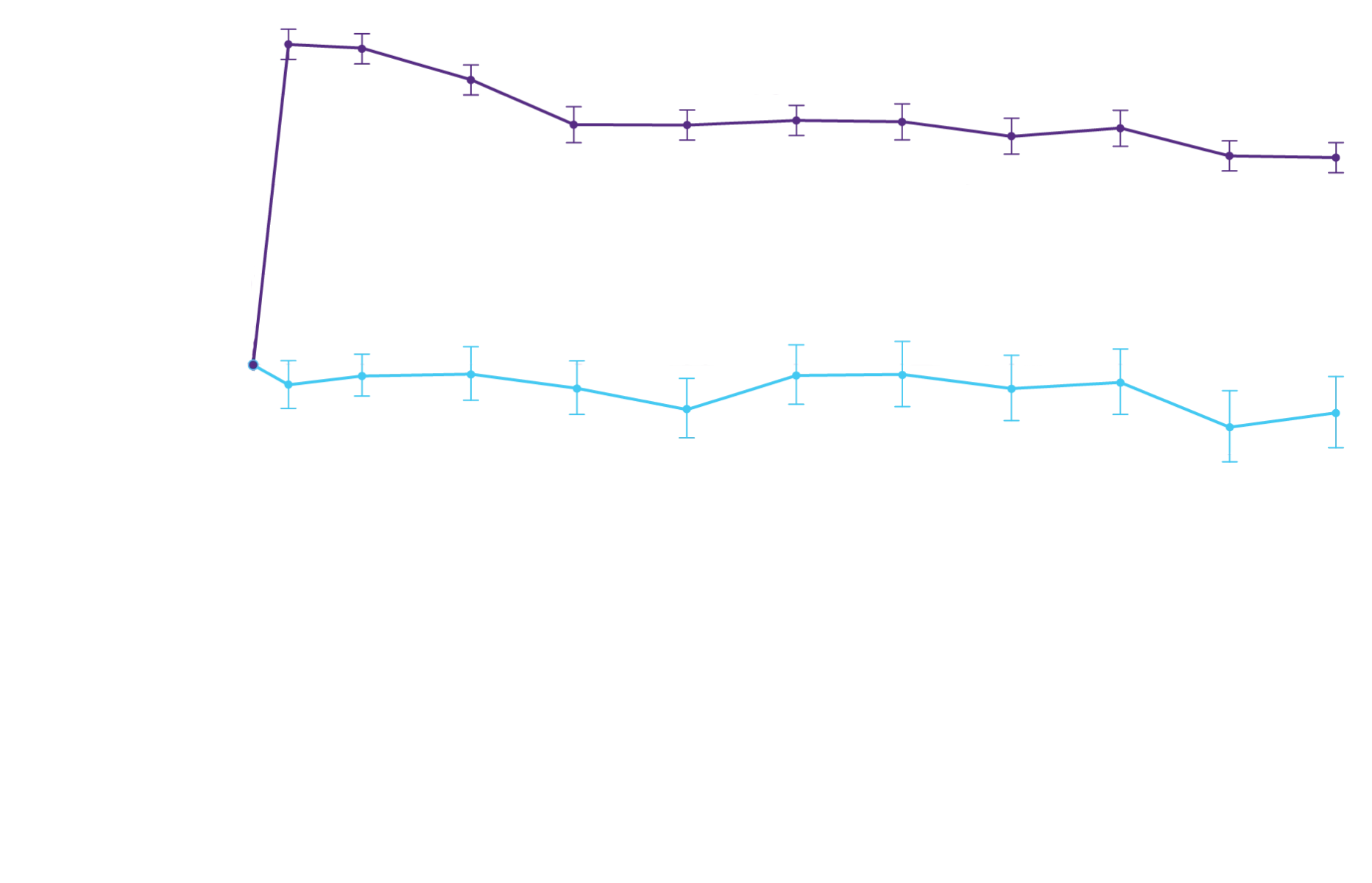
In a post hoc analysis,
in the composite of ACM and recurrent CVH3*
 Actor portrayals.
Actor portrayals.
*RRR was calculated using the negative binomial regression model. The total number of
events for Attruby compared with placebo (2:1 randomized) was
79 vs 52 for ACM,
respectively, and 182 vs 170 for CVH, respectively.3
6MWD=6-minute walk distance; ACM=all-cause mortality; CV=cardiovascular;
CVH=cardiovascular-related hospitalization; F-S test=Finkelstein-Schoenfeld test;
HR=hazard ratio; NT-proBNP=N-terminal pro–B-type natriuretic peptide; RRR=relative risk reduction.
References: 1. Attruby. Prescribing information. BridgeBio, Inc.; 2024. 2. Gillmore JD, Judge DP, Cappelli F, et al. Efficacy and safety of acoramidis in transthyretin amyloid cardiomyopathy. N Engl J Med. 2024;390(2):132-142. doi:10.1056/NEJMoa2305434 3. Judge DP, Alexander KM, Cappelli F, et al. Acoramidis improves clinical outcomes in patients with transthyretin amyloid cardiomyopathy: post hoc recurrent event analyses of ATTRibute-CM. Poster presented at: Heart Failure Society of America Annual Scientific Meeting; September 27-30, 2024; Atlanta, GA. 4. Data on file. BridgeBio, Inc.; 2024. 5. Gillmore JD, Judge DP, Cappelli F, et al. Efficacy and safety of acoramidis in transthyretin amyloid cardiomyopathy [study protocol]. N Engl J Med. 2024;390(2):132-142. doi:10.1056/NEJMoa2305434









_lines.webp)
_layout.webp)