Dosing + Safety
Attruby is conveniently dosed and has a safety profile demonstrated in patients with ATTR-CM
Actor portrayals.
Attruby tablets are available in a convenient 1-month supply1
The recommended dosage is 712 mg (two 356 mg tablets) taken
orally twice a day, with or without food
Tablets should be swallowed whole. Do not cut, crush, or chew

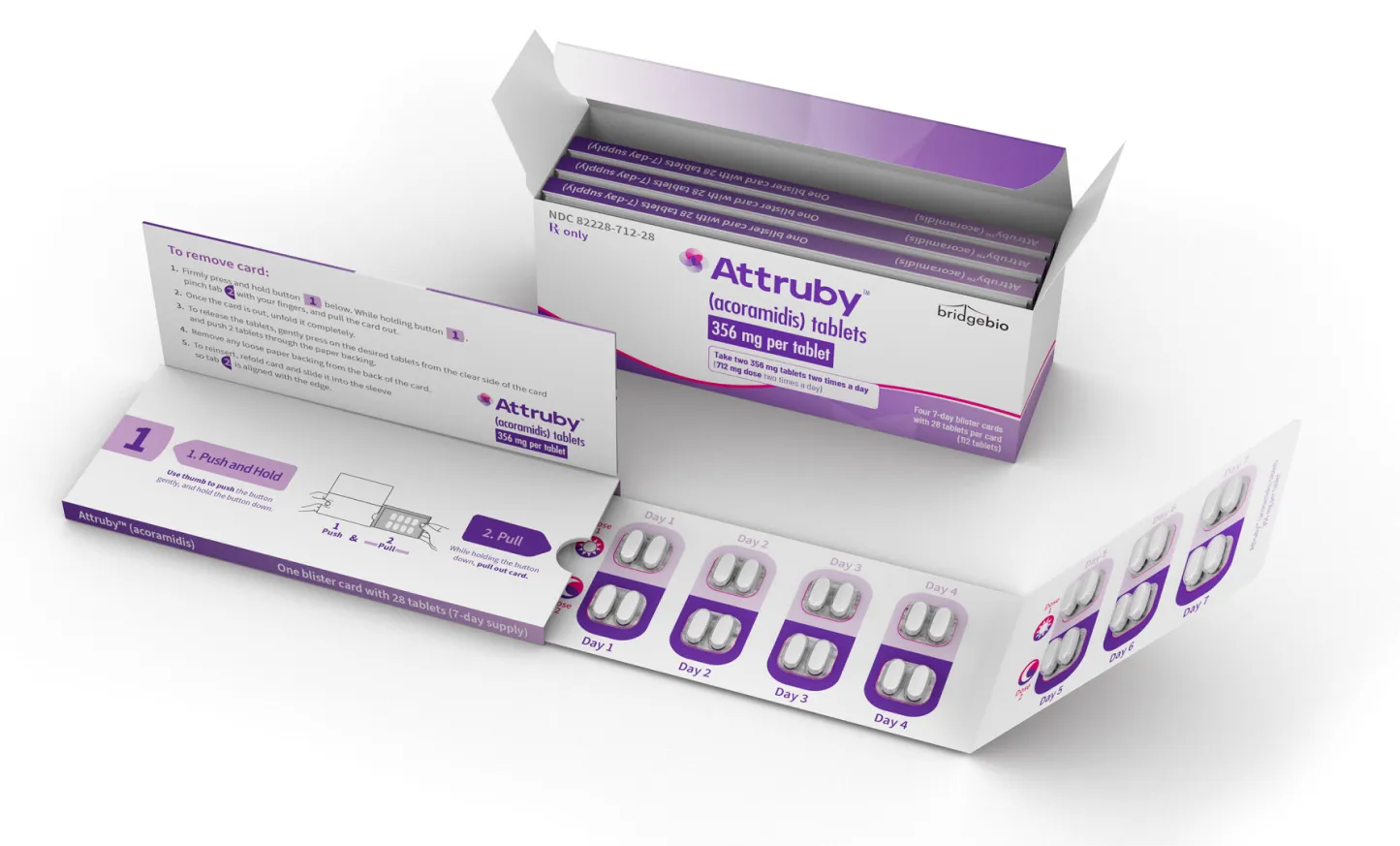
Not actual size.

Of patients with ATTR-CM,
9 out of 10 are already taking medications
multiple times a day2*
*Based on descriptive analyses of chronic oral medication use in patients from ATTRibute-CM (N=632) and real-world data of patients with ATTR-CM (N=4725) from Optum Clinformatics Data Mart.
ATTR-CM=transthyretin amyloid cardiomyopathy.
References: 1. Attruby. Prescribing information. BridgeBio, Inc.; 2024. 2. Data on file. BridgeBio, Inc.; 2024.
The safety profile of Attruby was demonstrated
in patients with ATTR-CM1
Safety outcomes from the ATTRibute-CM clinical trial*:

No
contraindications

There was a higher frequency of GI adverse reactions such as diarrhea 11.6% versus 7.6% and upper abdominal pain 5.5% versus 1.4% in the Attruby versus placebo group, respectively
Discontinuations due to adverse events were similar for Attruby (9.3%) and placebo (8.5%)

See more on the safety profile of Attruby
*Based on ITT population (632 patients). The ITT population was defined as all randomized participants who received at least 1 dose of study drug and had at least 1 postbaseline efficacy evaluation and included participants who had a baseline eGFR <30 mL/min/1.73 m2.2
ATTR-CM=transthyretin amyloid cardiomyopathy; eGFR=estimated glomerular filtration rate; GI=gastrointestinal; ITT=intent-to-treat.
References: 1. Attruby. Prescribing information. BridgeBio, Inc.; 2024. 2. Gillmore JD, Judge DP, Cappelli F, et al. Efficacy and safety of acoramidis in transthyretin amyloid cardiomyopathy. N Engl J Med. 2024;390(2): 132-142. doi:10.1056/NEJMoa2305434 3. Gillmore JD, Judge DP, Cappelli F, et al. Efficacy and safety of acoramidis in transthyretin amyloid cardiomyopathy. N Engl J Med. 2024;390(2)(suppl 1):1-34. doi:10.1056/NEJMoa2305434

Attruby tablets are available in a convenient 1-month supply1
The recommended dosage
is 712 mg (two 356 mg tablets)
taken
orally twice a day, with
or without food
Tablets should be swallowed
whole. Do not cut, crush, or chew


Not actual size.

Of patients with ATTR-CM,
9 out of 10 are already taking medications multiple times a day2*
*Based on descriptive analyses of chronic oral medication use in patients from ATTRibute-CM (N=632) and real-world data of patients with ATTR-CM (N=4725) from Optum Clinformatics Data Mart.
ATTR-CM=transthyretin amyloid cardiomyopathy.
References: 1. Attruby. Prescribing information. BridgeBio, Inc.; 2024. 2. Data on file. BridgeBio, Inc.; 2024.
The safety profile of Attruby was demonstrated in patients with ATTR-CM1
Safety outcomes from the ATTRibute-CM clinical trial*:

No
contraindications

There was a higher frequency of GI adverse reactions such as diarrhea 11.6% versus 7.6% and upper abdominal pain 5.5% versus 1.4% in the Attruby versus placebo group, respectively

Discontinuations due to adverse events were similar for Attruby (9.3%) and placebo (8.5%)

See more on the safety profile of Attruby
*Based on ITT population (632 patients). The ITT population was defined as all randomized participants who received at least 1 dose of study drug and had
at least 1 postbaseline efficacy evaluation and included participants who had a baseline eGFR
<30 mL/min/1.73 m2.2
ATTR-CM=transthyretin amyloid cardiomyopathy; eGFR=estimated glomerular filtration rate; GI=gastrointestinal; ITT=intent-to-treat.
References: 1. Attruby. Prescribing information. BridgeBio, Inc.; 2024. 2. Gillmore JD, Judge DP, Cappelli F, et al. Efficacy and safety of acoramidis in transthyretin amyloid cardiomyopathy. N Engl J Med. 2024;390(2): 132-142. doi:10.1056/NEJMoa2305434 3. Gillmore JD, Judge DP, Cappelli F, et al. Efficacy and safety of acoramidis in transthyretin amyloid cardiomyopathy. N Engl J Med. 2024;390(2)(suppl 1):1-34. doi:10.1056/NEJMoa2305434

Indication and Important safety information
INDICATION
Attruby® (acoramidis) is indicated for the treatment of the cardiomyopathy of wild-type or variant transthyretin-mediated amyloidosis (ATTR-CM) in adults to reduce cardiovascular death and cardiovascular-related hospitalization.
IMPORTANT SAFETY INFORMATION
Adverse Reactions
Diarrhea (11.6% vs 7.6%) and upper abdominal pain (5.5% vs 1.4%) were
reported in patients treated with Attruby versus placebo,
respectively. The majority of these adverse reactions were mild and
resolved without drug discontinuation.
Discontinuation rates due to adverse events were similar between patients treated with Attruby versus placebo (9.3% and 8.5%, respectively).
Laboratory Tests
Mean increase in serum creatinine of 0.2 and 0.0 mg/dL and a mean
decrease in eGFR of 8.2 and 0.7 mL/min/1.73 m2 was
observed in the
adults with ATTR-CM treated with Attruby versus placebo,
respectively, at Day 28 and then stabilized. These changes were
reversible after treatment discontinuation.
Use in Specific Populations
Pregnancy & Lactation: There are no data on the use of Attruby in pregnant women. Animal data have not shown developmental risk associated with the use of Attruby in pregnancy. There are no available data on the presence of Attruby in either human or animal milk or the effects of the drug on the breastfed infant or maternal milk production.
Please see Full Prescribing Information including Patient Information.
INDICATION AND IMPORTANT
SAFETY INFORMATION
INDICATION
Attruby® (acoramidis) is indicated for the treatment of the cardiomyopathy of wild-type or variant transthyretin-mediated amyloidosis (ATTR-CM) in adults to reduce cardiovascular death and cardiovascular-related hospitalization.
IMPORTANT SAFETY INFORMATION
Adverse Reactions
Diarrhea (11.6% vs 7.6%) and upper abdominal pain (5.5% vs 1.4%) were
reported in patients treated with Attruby versus placebo,
respectively. The majority of these adverse reactions were mild and
resolved without drug discontinuation.
Discontinuation rates due to adverse events were similar between patients treated with Attruby versus placebo (9.3% and 8.5%, respectively).
Laboratory Tests
Mean increase in serum creatinine of 0.2 and 0.0 mg/dL and a mean
decrease in eGFR of 8.2 and 0.7 mL/min/1.73 m2 was
observed in the
adults with ATTR-CM treated with Attruby versus placebo,
respectively, at Day 28 and then stabilized. These changes were
reversible after treatment discontinuation.
Use in Specific Populations
Pregnancy & Lactation: There are no data on the use of Attruby in pregnant women. Animal data have not shown developmental risk associated with the use of Attruby in pregnancy. There are no available data on the presence of Attruby in either human or animal milk or the effects of the drug on the breastfed infant or maternal milk production.
Please see Full Prescribing Information including Patient Information.
