Access & Support
ForgingBridges® is committed to
providing continuous support
Actor portrayals.
ForgingBridges is a personalized support program,
providing you and your patients with:

A dedicated team to support you, your office staff, and your patients
through the treatment journey
- Field Reimbursement Managers (FRMs) for healthcare providers and office staff
- Patient Access Liaisons (PALs) for patients and their caregivers
- ForgingBridges Specialists for personalized coverage support and financial assistance options

Insurance navigation
Regardless of insurance type, our dedicated ForgingBridges support team will help ensure all necessary
information has been collected, evaluate patients’ eligibility, and initiate next steps.

ForgingBridges Copay Assistance Program
Reduce out-of-pocket costs (including copays, coinsurance, or deductibles) to as little as $0 per month for Attruby for eligible, commercially insured patients.
Commercially insured patients only. Additional terms and conditions may apply
Once prescribed Attruby, patients or their caregivers can connect with ForgingBridges
Specialists who can screen their eligibility for financial assistance programs.
Questions? Call ForgingBridges at 1-888-55-BRIDGE (1-888-552-7434) Mon–Fri, 8 AM to 8 PM ET or visit ForgingBridges.com

ForgingBridges Free Trial
Provides eligible patients with a 1-month supply of Attruby.*
New Attruby patients with any insurance coverage

ForgingBridges Patient Assistance Program
May provide eligible patients with Attruby at no cost.
Patients must meet certain income criteria and be either currently uninsured or have limited coverage, or have received an appeal denial

ForgingBridges QuickStart
Provides a limited supply of Attruby at no cost for eligible patients experiencing coverage decision delays of 5 days or more.
Patients with any insurance type
*For eligibility criteria and terms and conditions, call ForgingBridges at 1-888-55-BRIDGE (1-888-552-7434) or visit ForgingBridges.com.

Questions?
Call ForgingBridges at:
1-888-55-BRIDGE
(1-888-552-7434)
Mon–Fri, 8 AM to 8 PM ET
Additional information on the
services offered by ForgingBridges is
available at ForgingBridges.com.
Find a variety of resources to help you, your patients,
and
office staff along the way
Office Staff Resources

Start Form
Use this form to prescribe Attruby for your appropriate patients and enroll them in the ForgingBridges®
Support Program.

Free Trial Program Form
Use this form to prescribe a 1-month supply of Attruby at no cost. New Attruby patients only.
Download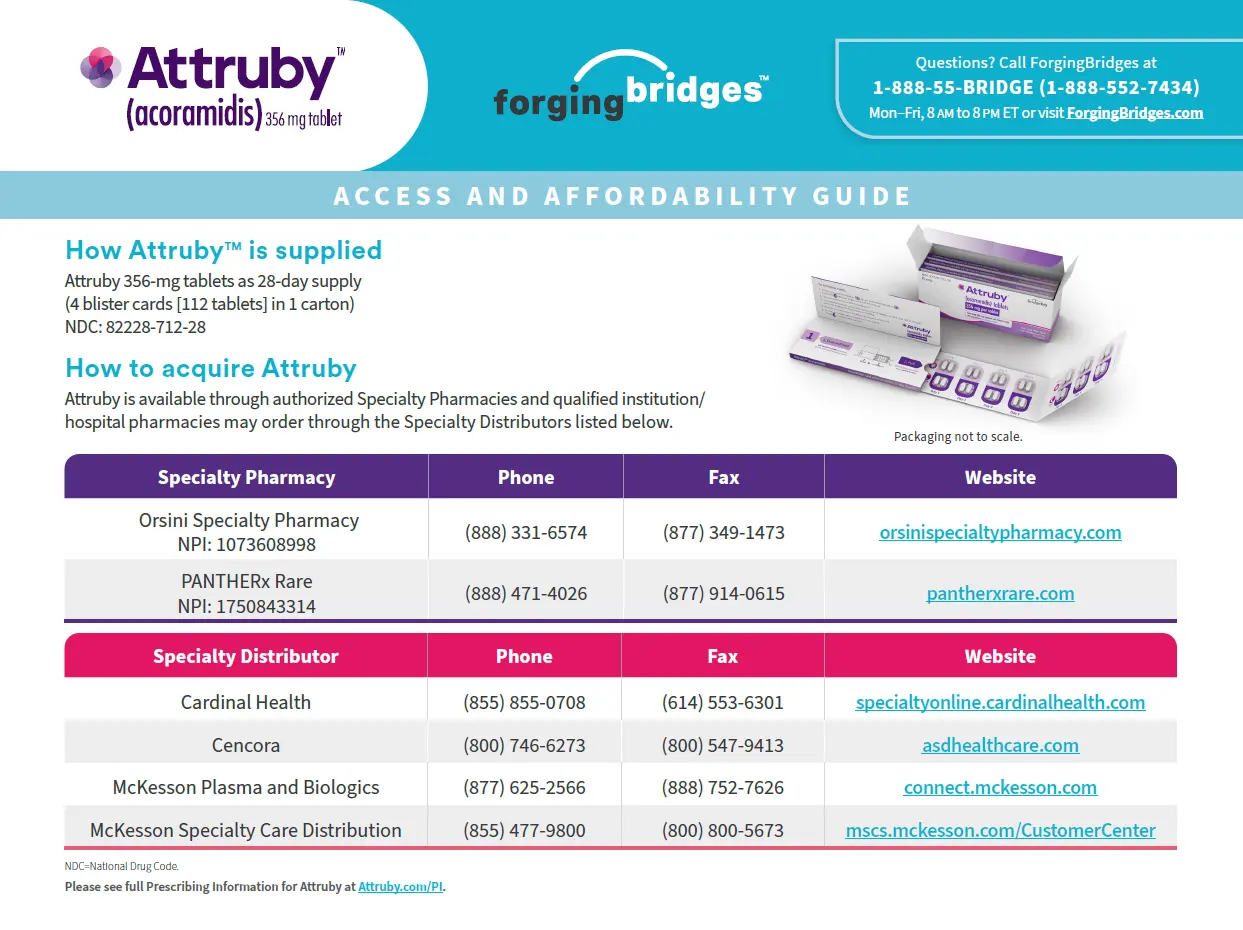
Access & Affordability Guide
Provides information on accessing Attruby and outlines affordability options available to eligible patients.
DownloadHCP ForgingBridges Support Video
Find out what support and resources may be available to your patients and office staff.
Watch NowPatient and Caregiver Resources

Medicare Part D Patient Guide
Educates patients with Medicare
Part D on the Medicare Prescription Payment Plan and eligibility for
low-income subsidy.
Patient Consent Form
Your patient may sign this standalone Patient Consent Form OR the patient section within the Attruby Start Form above to enroll in ForgingBridges.
Download
Commercial Copay Assistance Program Brochure
Provides your patients with information about the ForgingBridges Copay Assistance Program.
Download
ForgingBridges is a personalized support program, providing you
and your patients with:

A dedicated team
to support you, your
office
staff, and your
patients through the
treatment journey
- Field Reimbursement Managers (FRMs) for healthcare providers and office staff
- Patient Access Liaisons (PALs) for patients and their caregivers
- ForgingBridges Specialists for personalized coverage support and financial assistance options

Insurance navigation
Regardless of insurance type, our dedicated
ForgingBridges support team will help ensure all
necessary information has been collected, evaluate
patients’ eligibility, and initiate
next steps.

ForgingBridges Copay Assistance Program
Reduce out-of-pocket costs (including copays, coinsurance, or deductibles) to as little as $0 per month for Attruby for eligible, commercially insured patients.
Commercially insured patients only. Additional terms and conditions may apply
Once prescribed Attruby,
patients or their
caregivers can
connect with ForgingBridges
Specialists who can screen their
eligibility for financial
assistance programs.
Questions? Call ForgingBridges
at
1-888-55-BRIDGE (1-888-552-7434)
Mon–Fri, 8 AM to 8 PM ET or visit
ForgingBridges.com

ForgingBridges
Free Trial
Provides eligible patients with a 1-month supply of Attruby.*
New Attruby patients with any
insurance
coverage

ForgingBridges Patient Assistance Program
May provide eligible patients with Attruby
at no
cost.
Patients must meet certain income criteria and be either currently uninsured or have limited coverage, or have received an appeal denial

ForgingBridges QuickStart
Provides a limited supply of Attruby at no cost for eligible patients experiencing coverage decision delays of 5 days or more.
Patients with any insurance type
*For eligibility criteria and terms and conditions, call ForgingBridges at 1-888-55-BRIDGE (1-888-552-7434) or visit ForgingBridges.com.

Questions?
Call ForgingBridges at:
1-888-55-BRIDGE
(1-888-552-7434)
Mon–Fri, 8 AM to 8 PM ET
Additional information on
the
services offered by
ForgingBridges is
available
at ForgingBridges.com.
Find a variety of resources to help you, your
patients, and
office staff along
the way
Office Staff Resources

Start Form
Use this form to prescribe Attruby for your appropriate patients and enroll them in the ForgingBridgesTM Support Program.
Download
Free Trial Program Form
Use this form to prescribe a 1-month supply of Attruby at no cost. New Attruby patients only.
Download
Access & Affordability Guide
Provides information on accessing Attruby and outlines affordability options available to eligible patients.
DownloadHCP ForgingBridges Support Video
Find out what support and resources may be available to your patients and office staff.
Watch NowPatient and Caregiver Resources

Medicare Part D Patient Guide
Educates patients with Medicare
Part
D on the Medicare Prescription Payment
Plan and eligibility for
low-income subsidy

Patient Consent Form
Your patient may sign this standalone Patient Consent Form OR the patient section within the Attruby Start Form above to enroll in ForgingBridges
Download
Commercial Copay Assistance Program Brochure
Provides your patients with information about the ForgingBridges Copay Assistance Program.
Download

See the helpful
resources available to you, your staff,
and your patients
Indication and Important safety information
INDICATION
Attruby® (acoramidis) is indicated for the treatment of the cardiomyopathy of wild-type or variant transthyretin-mediated amyloidosis (ATTR-CM) in adults to reduce cardiovascular death and cardiovascular-related hospitalization.
IMPORTANT SAFETY INFORMATION
Adverse Reactions
Diarrhea (11.6% vs 7.6%) and upper abdominal pain (5.5% vs 1.4%) were
reported in patients treated with Attruby versus placebo,
respectively. The majority of these adverse reactions were mild and
resolved without drug discontinuation.
Discontinuation rates due to adverse events were similar between patients treated with Attruby versus placebo (9.3% and 8.5%, respectively).
Laboratory Tests
Mean increase in serum creatinine of 0.2 and 0.0 mg/dL and a mean
decrease in eGFR of 8.2 and 0.7 mL/min/1.73 m2 was
observed in the
adults with ATTR-CM treated with Attruby versus placebo,
respectively, at Day 28 and then stabilized. These changes were
reversible after treatment discontinuation.
Use in Specific Populations
Pregnancy & Lactation: There are no data on the use of Attruby in pregnant women. Animal data have not shown developmental risk associated with the use of Attruby in pregnancy. There are no available data on the presence of Attruby in either human or animal milk or the effects of the drug on the breastfed infant or maternal milk production.
Please see Full Prescribing Information including Patient Information.
INDICATION AND IMPORTANT
SAFETY INFORMATION
INDICATION
Attruby® (acoramidis) is indicated for the treatment of the cardiomyopathy of wild-type or variant transthyretin-mediated amyloidosis (ATTR-CM) in adults to reduce cardiovascular death and cardiovascular-related hospitalization.
IMPORTANT SAFETY INFORMATION
Adverse Reactions
Diarrhea (11.6% vs 7.6%) and upper abdominal pain (5.5% vs 1.4%) were
reported in patients treated with Attruby versus placebo,
respectively. The majority of these adverse reactions were mild and
resolved without drug discontinuation.
Discontinuation rates due to adverse events were similar between patients treated with Attruby versus placebo (9.3% and 8.5%, respectively).
Laboratory Tests
Mean increase in serum creatinine of 0.2 and 0.0 mg/dL and a mean
decrease in eGFR of 8.2 and 0.7 mL/min/1.73 m2 was
observed in the
adults with ATTR-CM treated with Attruby versus placebo,
respectively, at Day 28 and then stabilized. These changes were
reversible after treatment discontinuation.
Use in Specific Populations
Pregnancy & Lactation: There are no data on the use of Attruby in pregnant women. Animal data have not shown developmental risk associated with the use of Attruby in pregnancy. There are no available data on the presence of Attruby in either human or animal milk or the effects of the drug on the breastfed infant or maternal milk production.
Please see Full Prescribing Information including Patient Information.
